Periorbital Complications of Radiation Therapy
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
Introduction
Radiation to the periocular region is routinely used as a curative, adjuvant, neoadjuvant, or palliative regimen for eyelid cancers, intraocular cancers, orbital tumors, or autoimmune diseases.[1] Radiation therapy (also known as radiotherapy) is performed through an internal or external beam to damage the DNA of cells and trigger cell apoptosis. External beam radiotherapy uses an external source of ionizing radiation targeting a specific site on the body. Internal beam radiotherapy (also known as brachytherapy) places a sealed source (plaque, seed, or wire) into or adjacent to the site of malignancy or by injecting an unsealed source of therapeutic radioisotope, which localizes to the tissue requiring destruction. While significant advances have been made to localize and contain radiation treatment to the target tissue, there is still a risk of exposure to adjacent healthy tissue. This inadvertent exposure leads to acute and chronic complications in the orbit and periorbita. [2]
Acute complications to periocular tissue, such as edema and vascular congestion, can present within the weeks of starting radiation treatment.
Chronic complications of fibrosis, scarring, vascular injury, atrophy, nerve injury, and secondary malignancies can occur in the months to years after completion of radiation therapy.[3]
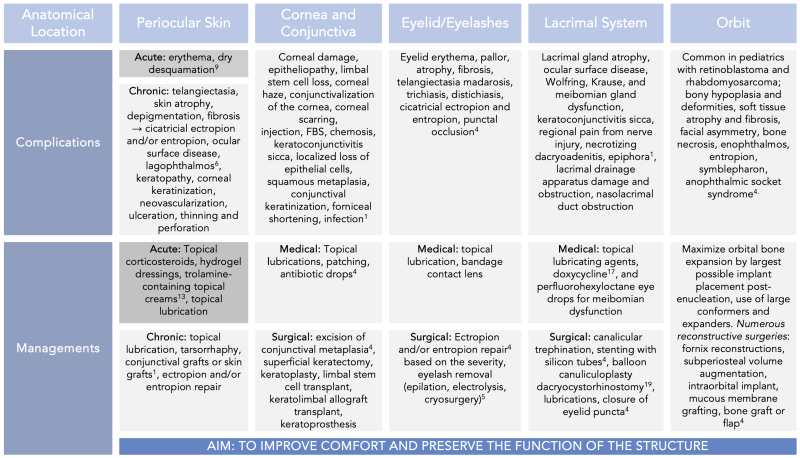
The most affected periorbital structures after radiation therapy are the eyelids (periorbital skin, eyelashes, and glands), lacrimal system (lacrimal gland and drainage system), orbit and soft tissue, and cranial nerves.[1] This article discusses oculoplastic complications of radiation therapy and management options.
Eyelid
Eyelid Structures
The eyelid is composed of:
- Skin and subcutaneous tissue – the most delicate and thinnest skin in the body. The dermis of the skin contains hair follicles, sebaceous and sweat glands, blood vessels, and nerves.
- Muscles and Fascia: Protractor muscle (orbicularis oculi)
- Superior retractor muscles (levator palpebrae superioris and levator aponeurosis, Müller’s muscle)
- Inferior retractors (capsulopalpebral fascia and inferior tarsal muscle)
- Orbital septum
- Orbital fat pads
- Tarsus
- Glands (Meibomian, Wolfring, Krause)
- Palpebral conjunctiva.
Skin and Eyelashes
Radiation therapy (RT) to the eyelid most commonly affects the eyelid skin, hair follicles, glands, and the palpebral conjunctiva.[1] Skin changes commonly observed after RT include eyelid erythema followed by pallor or hyperpigmentation, atrophy, and telangiectasia. Skin reactions of erythema and crusting typically peak within 10-20 days and resolve within 2 to 4 weeks. Complete healing is anticipated by eight weeks. Chronic structural changes can also occur months to years after therapy.[4] Atrophy or fibrosis of the periocular skin can lead to upper or lower eyelid cicatricial ectropion if involving the anterior lamella and cicatricial entropion if affecting the posterior lamella.[4]
RT-associated injury to the hair follicle can result in madarosis (loss of eyelash) or aberrant growth (trichiasis and distichiasis). Dermal changes from radiation exposure can affect the position of the hair follicle, which results in eyelash growth towards the globe. Acquired distichiasis from radiation exposure causes eyelash growth from the meibomian gland orifice.[1] For both trichiasis and distichiasis, patients experience foreign body sensation, tearing, photophobia, and discomfort.
The glands of the eyelid are discussed in the section "Lacrimal System and Glands of the Eye (Meibomian, Krause, and Wolfring).”
Management
Medical management
Ocular surface irritation can occur from trichiasis, distichiasis, entropion, ectropion, and lagophthalmos. The first-line management of eyelid complications from RT includes conservative treatment for ocular surface irritation. Treatment includes manual epilation, topical lubrication, and bandage contact lens placement for comfort.
Surgical Management
Trichiasis and Distichiasis
- Traditional surgical management includes inter-marginal split lamella with graft or lid lamella resection. Alternate therapies include electrolysis (advantageous for localized trichiasis or distichiasis, disadvantageous for lanugo hair) or cryotherapy of the eyelash root.[5] Buccal membrane grafting is typically reserved for refractive cases.
Cicatricial entropion repair
- Mild: skin resection to rotate the lid margin
- Moderate: external partial tarsectomy, full-thickness tarsectomy with everting sutures, transverse blepharotomy
- Severe: lengthening of the posterior lamella with release of scar tissue. Lengthening can be done with an ear cartilage graft, hard palate graft, or allograft.
Cicatricial ectropion repair
- Involves lengthening of the anterior lamellae with a skin graft
Periocular Skin
Periocular skin is highly radiosensitive due to its rapid cell turnover. The onset and severity of the skin complications are dose-dependent. Skin damage from radiation therapy is typically the earliest and most common complication. Risk factors include:
- Male gender
- Older age
- Prior sun exposure
- Systemic comorbidities (Diabetes Mellitus or Collagen Vascular Diseases)
Acute complications
The earliest sign of skin damage is erythema, which can present within 24 hours of radiation therapy. This is followed by dry desquamation, which can take 2 to 4 weeks to resolve after completing treatment. [8][9] A study showed that even a relatively smaller dose of <1.5 Gy per fraction ubiquitously resulted in acute erythema.[10]
Chronic complications
Months to years after completing radiation therapy, the periocular skin can undergo skin atrophy, depigmentation or hyperpigmentation, fibrosis, and develop telangiectasias.[6] The long-term effect of radiation can also result in cicatricial changes from premature terminal differentiation of fibroblasts. This causes significant collagen deposition over many months and shortens the anterior lamella. Subsequent traction to the upper and/or lower eyelid skin occurs, leading to a cicatricial ectropion. Cicatricial ectropion exposes the globe to the atmosphere, resulting in ocular surface disease and lagophthalmos with a high risk of more severe complications such as keratopathy, corneal keratinization, corneal neovascularization, corneal ulceration, and corneal thinning with possible perforation.
Management
The management of periocular skin complications from radiation therapy typically includes topical corticosteroids for their combined anti-inflammatory, immunosuppressive, anti-proliferative, and vasoconstrictive effects.[11] This agent is, however, typically avoided in the delicate eyelid skin. Hydrogel dressings and trolamine-containing topical creams are also widely used.[12]
The first line treatment for ocular surface disease due to exposure is topical lubrication. This is followed by lid taping and then placement of a temporary tarsorrhaphy to improve eyelid closure. More severe exposure-related complications can benefit from amniotic membrane grafts. For severe cicatricial changes from periocular skin contraction, surgical intervention may be required to release the fibrotic tissue and lengthen the anterior skin with a full-thickness skin graft.[1] It is important to note that periocular skin changes from RT can also include eyelid skin changes. Therefore, cicatricial ectropion of the eyelid can also be present. Management for eyelid cicatricial ectropion is discussed under “Eyelid, subsection: surgical management.”
Lacrimal System and Glands of the Eye (Meibomian, Krause, and Wolfring)
The lacrimal system is comprised of the lacrimal gland, the upper and lower puncta, canaliculi, common canaliculus, lacrimal sac, and the nasolacrimal duct. The lacrimal gland is an exocrine gland with two lobes connected by interlobular ducts.[13] Accessory lacrimal glands are the Krause and Wolfring glands located in the conjunctival fornices and the nonmarginal borders of the tarsal plate, respectively.[14] The lacrimal gland produces a significant component of the aqueous layer in tears and is rich in antibodies, antimicrobial agents, and growth factors. The meibomian glands are holocrine glands located in the tarsal plate of both the upper and lower eyelid and are the source of the lipid layer of tears.[15]
Injury to the lacrimal system and glands can occur with any radiation exposure when treating tumors of the lacrimal gland, orbit, or adnexa, in addition to RT for orbital inflammatory diseases such as orbital pseudotumor, myositis, and thyroid eye disease. Damage to the lacrimal system and glands of the eye is cumulative and dose-dependent.
After exposure to radiation, histopathology of the lacrimal gland can show extensive serous acinar damage, reduction in the size and number of serous acini (indicative of lacrimal gland atrophy), and necrosis. The earliest reported changes in literature to the lacrimal gland were within 48 hours after the first dose of radiation therapy, and lacrimal gland atrophy was noted to be present within two days of radiation exposure.[3] Clinically, this can present with poor tear film composition (reduced aqueous component) and decreased tear film production. This can lead to ocular surface disease and the development of keratoconjunctivitis sicca. Additionally, patients can also have regional pain in affected areas due to nerve-related injury. Any symptomatic patient should be monitored for the development of necrotizing dacryoadenitis.[1]
Similarly, meibomian, Krause and Wolfring glands can also have significant injury from radiation exposure leading to partial or complete loss of function. Clinical symptoms of meibomian gland dysfunction or complete loss of function can be exacerbated if there is presence of blepharitis. Loss of meibomian gland function causes a decrease in the lipid layer of the tear film, leading to a more evaporative etiology for ocular surface disease. Loss of Krause or Wolfring gland function would result in a decrease in the aqueous portion of tears.
If the lacrimal drainage apparatus (canalicular system or lacrimal sac) is affected by treatment, it can lead to tear outflow obstruction from reactive adhesions, stenosis, and scarring anywhere from the punctal orifice through the valve of Hasner. Proximal injury can lead to fibrotic narrowing and canalicular stenosis. If the injury is more distal (starting from the lacrimal sac), it is an acquired nasolacrimal duct obstruction (NLDO). Clinical presentation of NLDO includes epiphora, foreign body sensation, blurry vision, ocular fatigue, mucopurulent discharge, pain, and dacryocystitis.
Management
Medical Management
Treatment for ocular surface disease from lacrimal, meibomian, Krause, or Wolfring gland can be treated based on extent of damage, and type of tear component reduction. If there is functional loss of the lacrimal, Krause, or Wolfring glands, the reduction of aqueous in the tear can be supplemented with topical lubricating agents (tears, gel, and/or ointments). If there is partial function loss of the meibomian gland, low-dose oral doxycycline can be used to stabilize the tear film by suppressing bacterial lipases and decreasing inflammation by reducing pro-inflammatory molecules such as free fatty acids.[16] If there is a complete loss of meibomian gland function, then the lipid component can be supplemented with perfluorohexyloctane ophthalmic solution (trade name: Meibo), which mimics the natural meibum and reduces evaporative dry eye disease.
Surgical Management
- For mild cases, proximal partial obstruction of the canalicular system can be managed with reconstruction. Trephination of scarred tissue followed by stenting or balloon canuliculoplasty can be used to reconstruct a new lumen.[17]
- Outflow obstruction from partial or full obstruction of the common canaliculus and more distal structures can be managed with dacryocystorhinostomy (DCR). Studies have shown that DCR successfully alleviates nasolacrimal duct obstruction in most patients after head and neck radiation.[18][19] Severe canalicular and nasal mucosal scarring are possible reasons for DCR failure. [19]
Orbit
The orbit is composed of the bony orbit, orbital septum, extraocular muscles, cranial nerves, vasculature, the lacrimal gland, and fat. The bony orbit is comprised of the orbital roof (frontal bone and lesser wing of the sphenoid bone), medial orbital wall (maxillary bone, lacrimal bone, ethmoid bone, and the lesser wing of the sphenoid), orbital floor (maxillary bone, palatine bone, and zygomatic bone), lateral orbital wall (zygomatic bone and greater wing of the sphenoid).
Amongst the pediatric population, radiation is most often used to treat retinoblastoma or rhabdomyosarcoma. Adult and pediatric patients can also receive orbital RT for orbital inflammatory disorders such as orbital pseudotumor (idiopathic orbital inflammatory syndrome) and thyroid-associated orbitopathy.
Radiation-induced orbital changes vary based on patient age. Radiation exposure in the pediatric population is unique because it can cause stunted bone growth. This can lead to hypoplasia of the face, orbit, and eyelid(s), resulting in facial asymmetry. In adults, radiation causes orbital soft tissue atrophy and fibrosis, orbital bone atrophy, or malformation, which can also lead to facial asymmetry and enophthalmos. All effects of radiation therapy are dose dependent. Hence, high dose radiation therapy has the risk of causing bone necrosis. Additionally, both pediatric and adult patients with an anophthalmic socket can develop post-enucleation socket syndrome (PESS) or Contracted Socket Syndrome.
High-power X-ray beams injure osteocytes, osteoblasts, and osteoclasts, affecting bony remodeling. The degree of bony deformity and inhibition of bone growth has been found to be more significant in younger patients. Vascular injury has also been noted, and this results in tissue hypoxia leading to soft tissue damage, atrophy, and fibrosis.[4][20] Poor bone remodeling and decreased nutritional status of the structures and tissues collectively lead to poor development of the orbital complex (due to decreased vertical and horizontal diameters), hypoplasia of the nasal bridge, zygomatic bone, and the temporal fossa. Hypoplasia of the midface structures are juxtaposed to the normal growth that still occurs on the tip of the nose, nasal alae, and the frontal bone causing the deformities to appear more disproportionate and prominent. A study by Raney et. al. observed that 59% of all pediatric patients treated with RT for orbital rhabdomyosarcoma developed orbital hypoplasia.[21] Similarly, a study by Gevorgyan et al. found that 66-100% of all children in the study who received RT for any head and neck cancer developed profound craniofacial bony anomalies.[20]
Enophthalmos can occur with malformation of the orbital bones (from hypoplasia or atrophy) and orbital soft tissue injury which causes fat atrophy and fibrosis. Fibrosis leads to tethering and retraction of the globe posteriorly. Enophthalmos was present in 4 out of 12 pediatric patients with RT for orbital rhabdomyosarcoma after receiving 6000 cGy of radiation divided into 200cGy fractions.[22] Notably, a dose of 2000 to 3750 cGy of radiation divided into 200-250cGy fractions has been shown to lead to fewer soft tissue complications when treating for pseudotumor, orbital lymphoma, and thyroid orbitopathy.
Anophthalmic socket syndrome (also known as Post-Enucleation Socket Syndrome or PESS) can occur in any post-radiation socket without a globe, causing stretching of the socket structures. This can present with pseudoptosis or upper eyelid retraction, deep superior sulcus, shallow inferior sulcus, lower eyelid laxity causing ocular surface disease, and posterior displacement of the prosthesis. The malformation of the socket results in facial asymmetry and can have a negative psychosocial impact on a patient. Contracted socket syndrome is another similar adverse effect of radiation therapy in an anophthalmic socket, and it is distinguished from PESS by the inability of the socket to support a prosthesis due to shortened soft tissue and diminished or obliterated fornices.[4] This is due to cicatricial conjunctival changes.[23]
Management
The management of pediatric orbital changes from radiation therapy focuses on promoting normal socket, eyelid, and facial development. The force from adjacent orbital tissue stimulates pediatric orbital and facial bone development. The crux of the management is to maximize orbital bone expansion as a child develops. This can be done by placing the largest implant after an enucleation, continually increasing conformer size, and utilizing expanders (hydrogel, silicone, or hydrophilic).
Enophthalmos with a difference of more than 2 mm on exophthalmometry is clinically significant. It can be managed with orbital reconstruction, using a sheet implant along the medial wall and orbital floor to restore the orbital structure.
Surgical management of PESS and Contracted Socket Syndrome aims to reconstruct the fornices, improve orbital volume, and mitigate contracture. Reconstruction of fornices depends on the severity and extent of scarring. Excision of symblepharon, followed by a local flap, horizontal tightening procedures, externalized conjunctival sutures, or conjunctival fixation to the inferior orbital rim periosteum, are all potential techniques utilized on a case-by-case basis. If the shortened fornix is due to scarring, such as in Contracted Socket Syndrome, the posterior lamella can be lengthened using mucosal grafts (buccal or nasal septum), ear cartilage grafts, or hard palate grafts. Contractures can be treated with split-thickness mucosal grafts, full-thickness conjunctival autografts, or amniotic membrane grafts. Volume loss can be addressed with dermis fat grafting. Management of severe contractures can be complex. Options include space-occupying alloplastic material or conformers fixed to the orbital rim. Further reconstruction can be done with microvascular free flaps by co-management with a general plastic surgery service.[1][4][23]
Bone necrosis due to high-dose radiation exposure requires complex, multidisciplinary management. Reconstruction of the bone can be done with a bone graft (relies on the blood supply of the recipient wound bed) or bone flap (relies on microvascular anastomoses). However, due to poor vasculature and diminished adjacent tissue health, there is a high risk of infection and failure of the bone graft.
Cornea and Conjunctiva
The ocular surface is comprised of the cornea and the conjunctiva. The cornea is a transparent, avascular structure composed of the epithelium, Bowman’s layer, stroma, Descemet’s membrane, Dua’s layer, and the endothelium. At the peripheral limbus of the cornea, there are unipotent ocular stem cells (limbal stem cells) within the palisades of Vogt. The conjunctiva is composed of a non-keratinized epithelium layer with goblet cells and stratified squamous cells. Deeper to the epithelium is the submucosa of the conjunctiva, which consists of superficial lymphoid and fibrous tissue.[24]
Radiation therapy to the cornea and conjunctiva is dose-dependent. Corneal damage occurs from injury to the corneal epithelium all the way to the endothelium. As corneal transparency and avascularity are dependent on the continued replacement of devitalized corneal cells, RT damage to the limbal stem cell can cause a loss of the stem cells. This can lead to recurrent epithelial erosions, progressive epitheliopathy, increasing corneal haze, corneal conjunctivalization, and scarring. This is further exacerbated by RT exposure to the conjunctiva, lacrimal glands, and eyelid glands, leading to a collective effect on the ocular surface from poor tear film and quantity.
Conjunctival changes from RT are similar to periocular skin changes seen with radiation exposure, as well as other disease processes causing chronic irritation and inflammation of the ocular surface. Clinically, this presents with foreign body sensation, blurry vision, epiphora, mucus stranding, keratinization, and corneal neovascularization. Acutely, injection and chemosis are more prominently observed after radiation therapy. In the long term, keratoconjunctivitis sicca can develop due to ocular surface irregularity, poor tear composition, and reduced tear formation. Other reported complications are localized loss of epithelial cells, squamous metaplasia, conjunctival keratinization, and forniceal shortening.[1]
Management
Medical management of corneal damage from RT includes aggressive topical lubrication, bandage contact lenses or scleral contact lenses for comfort, lid hygiene, and warm compresses to optimize other components that affect the ocular surface. Conjunctival complications can be managed with topical lubricating agents, patching, and antibiotic drops. In the case of squamous metaplasia of the conjunctiva, excision is recommended.[1]
Surgical management of the cornea depends on the location and extent of damage. Management is typically done by the cornea service. Treatment options range from superficial keratectomy to penetrating keratoplasty. Treatment of limbal stem cell deficiency includes autologous or allogeneic transplant, ex vivo cultivation of limbal stem cells, keratolimbal allograft transplantation, or keratoprosthesis.
Summary
Radiation exposure to periocular structures can cause a wide range of dose-dependent damage. Severity is also dependent on the location and proximity of the radiation source and the patient's health status. Management of these complications is individualized and aimed at providing comfort, with a primary focus on preserving vision and functionality.
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 1.6 1.7 1.8 1.9 Connor M, Esmaeli B, Ch39: Oculoplastic Complications of Cancer Therapy. In: Black EH, Nesi FA, Calvano CJ, Gladstone GJ, Levine MR, editors. Smith and Nesi’s Ophthalmic Plastic and Reconstructive Surgery. Vol Third edition. Springer; 2012.
- ↑ Barabino S, Raghavan A, Loeffler J, Dana R. Radiotherapy-induced ocular surface disease. Cornea. 2005 Nov;24(8):909-14. doi: 10.1097/01.ico.0000154235.64359.d3. PMID: 16227831.
- ↑ 3.0 3.1 Stephens LC, Schultheiss TE, Peters LJ, Ang KK, Gray KN. Acute Radiation Injury of Ocular Adnexa. Arch Ophthalmol. 1988;106(3):389–391. doi:10.1001/archopht.1988.01060130415032
- ↑ 4.0 4.1 4.2 4.3 4.4 4.5 Gordon KB, Char DH, Sagerman RH. Late effects of radiation on the eye and ocular adnexa. Int J Radiat Oncol Biol Phys. 1995 Mar 30;31(5):1123-39. doi: 10.1016/0360-3016(95)00062-4. PMID: 7713778.
- ↑ Singh S. Distichiasis: An update on etiology, treatment and outcomes. Indian J Ophthalmol. 2022 Apr;70(4):1100-1106. doi: 10.4103/ijo.IJO_1141_21. PMID: 35325995; PMCID: PMC9240497.
- ↑ 6.0 6.1 Durkin SR, Roos D, Higgs B, Casson RJ, Selva D. Ophthalmic and adnexal complications of radiotherapy. Acta Ophthalmologica Scandinavica. 2006;85(3):240-250
- ↑ Parsons JT, Bova FJ, Mendenhall WM, Million RR, Fitzgerald CR. Response of the normal eye to high-dose radiotherapy. Oncology. 1996 Jun;10(6):837-47; discussion 847-8, 851-2. PMID: 8823799.
- ↑ Simonen P, Hamilton C, Ferguson S, Ostwald P, O'Brien M, O'Brien P, Back M, Denham J. Do inflammatory processes contribute to radiation-induced erythema observed in the skin of humans? Radiother Oncol. 1998 Jan;46(1):73-82. doi: 10.1016/s0167-8140(97)00115-1. PMID: 9488130.
- ↑ Nuzzi R, Trossarello M, Bartoncini S, Marolo P, Franco P, Mantovani C, Ricardi U. Ocular Complications After Radiation Therapy: An Observational Study. Clin Ophthalmol. 2020 Oct 9;14:3153-3166. doi: 10.2147/OPTH.S263291. PMID: 33116366; PMCID: PMC7555281.
- ↑ Hamilton CS, Denham JW, O'Brien M, Ostwald P, Kron T, Wright S, Drr W. Underprediction of human skin erythema at low doses per fraction by the linear quadratic model. Radiother Oncol. 1996 Jul;40(1):23-30. doi: 10.1016/0167-8140(96)01764-1. PMID: 8844884.
- ↑ Meghrajani CF, Co HC, Ang-Tiu CM, Roa FC. Topical corticosteroid therapy for the prevention of acute radiation dermatitis: a systematic review of randomized controlled trials. Expert Rev Clin Pharmacol. 2013 Nov;6(6):641-9. doi: 10.1586/17512433.2013.841079. PMID: 24164612.
- ↑ Wei J, Meng L, Hou X, Qu C, Wang B, Xin Y, Jiang X. Radiation-induced skin reactions: mechanism and treatment. Cancer Manag Res. 2018 Dec 21;11:167-177. doi: 10.2147/CMAR.S188655. PMID: 30613164; PMCID: PMC6306060.
- ↑ Machiele R, Lopez MJ, Czyz CN. Anatomy, Head and Neck: Eye Lacrimal Gland. [Updated 2023 Jul 24]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK532914/
- ↑ Conrady CD, Joos ZP, Patel BC. Review: The Lacrimal Gland and Its Role in Dry Eye. J Ophthalmol.2016;2016:7542929. doi: 10.1155/2016/7542929. Epub 2016 Mar 2. PMID: 27042343; PMCID: PMC4793137.
- ↑ Foulks GN, Ch10: Meibomian Gland Disease: Treatment. In: Holland EJ, Mannis MJ, Lee B, editors. Ocular Surface Disease: Cornea, Conjunctiva and Tear Film. Elsevier; 2013
- ↑ Sabeti S, Kheirkhah A, Yin J, Dana R. Management of meibomian gland dysfunction: a review. Surv Ophthalmol.2020 Mar-Apr;65(2):205-217. doi: 10.1016/j.survophthal.2019.08.007. Epub 2019 Sep 5. PMID: 31494111.
- ↑ Canalicular Obstruction. American Academy of Ophthalmology, EyeWiki. https://eyewiki.aao.org/Canalicular_Obstruction. Accessed March 30, 2024.
- ↑ Diba R, Saadati H, Esmaeli B. Outcomes of dacryocystorhinostomy in patients with head and neck tumors. Head Neck. 2005 Jan;27(1):72-5. doi: 10.1002/hed.20079. PMID: 15565560.
- ↑ 19.0 19.1 El-Sawy T, Ali R, Nasser QJ, Esmaeli B. Outcomes of dacryocystorhinostomy in patients with head and neck cancer treated with high-dose radiation therapy. Ophthalmic Plast Reconstr Surg. 2012 May-Jun;28(3):196-8. doi: 10.1097/IOP.0b013e31824c11df. PMID: 22460683; PMCID: PMC3878067.
- ↑ 20.0 20.1 Gevorgyan A, La Scala GC, Neligan PC, Pang CY, Forrest CR. Radiation-induced craniofacial bone growth disturbances. J Craniofac Surg. 2007 Sep;18(5):1001-7. doi: 10.1097/scs.0b013e31812f7584. PMID: 17912072.
- ↑ Raney RB, Anderson JR, Kollath J, Vassilopoulou-Sellin R, Klein MJ, Heyn R, Glicksman AS, Wharam M, Crist WM, Maurer HM. Late effects of therapy in 94 patients with localized rhabdomyosarcoma of the orbit: Report from the Intergroup Rhabdomyosarcoma Study (IRS)-III, 1984-1991. Med Pediatr Oncol. 2000 Jun;34(6):413-20. doi: 10.1002/(sici)1096-911x(200006)34:6<413::aid-mpo6>3.0.co;2-4. PMID: 10842248.
- ↑ Fiorillo A, Migliorati R, Grimaldi M, Vassallo P, Canale G, Tranfa F, Uccello G, Fiore M, Muto P, Menna G, et al. Multidisciplinary treatment of primary orbital rhabdomyosarcoma. A single-institution experience. Cancer. 1991 Feb 1;67(3):560-3. doi: 10.1002/1097-0142(19910201)67:3<560::aid-cncr2820670305>3.0.co;2-t. PMID: 1985749.
- ↑ 23.0 23.1 Contracted socket. American Academy of Ophthalmology, EyeWiki. https://eyewiki.aao.org/Contracted_Socket.Accessed March 30, 2024.
- ↑ Sridhar MS. Anatomy of cornea and ocular surface. Indian J Ophthalmol. 2018 Feb;66(2):190-194. doi: 10.4103/ijo.IJO_646_17. PMID: 29380756; PMCID: PMC5819093.