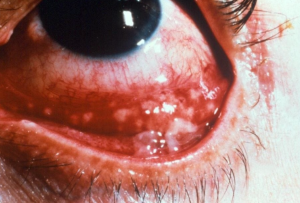
Parinaud Oculoglandular Syndrome
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
Disease Entity
Parinaud oculoglandular syndrome is a conjunctival disease characterized by a unilateral granulomatous follicular conjunctivitis associated with ipsilateral regional lymphadenopathy. There are many vectors that transmit organisms that cause oculoglandular syndrome, of which, Cat Scratch Disease is the most common cause. Prognosis in most cases is excellent. [1]
Diagnostic testing and treatment should be dictated by patient history, clinical findings, and symptoms.
Etiology[1]
|
Most common | |
|---|---|
|
Bartonella henselae | |
|
More common | |
|
Tularemia |
Francisella tularensis |
|
Sporotrichosis |
Sporothrix schenckii |
|
Less common | |
|
Tuberculosis |
Mycobacterium tuberculosis |
|
Coccidioidomycosis |
Coccidioides immitis |
|
Syphilis |
Treponema pallidum |
|
Rare | |
|
Herpes Simplex |
Herpes virus |
|
Sarcoidosis |
|
|
Chancroid |
Haemophilus ducreyi |
|
Pasteurellosis |
Pasteurella multocida |
|
Yersinia sp. |
Yersinia enterocolitica Yersinia pseudotuberculosis |
|
Hansen’s disease |
Mycobacterium leprae |
|
Listeria |
Listeria monocytogenes |
|
Mumps |
Mumps virus |
|
Infectious mononucleosis |
Epstein-Barr virus |
|
Blastomycosis |
Blastomyces dermatitidis |
|
Mediterranean fever |
Rickettsia conorii |
|
Vaccinia |
Smallpox vaccine |
|
Lymphogranuloma Venereum (LGV) |
Chlamydia trachomatis-LGV subtype |
|
Paracoccidioidomycosis |
Paracoccidioides brasiliensis |
|
Glanders |
Burkholderia mallei |
General Pathology
Cat-Scratch Disease
Cat-scratch disease (CSD) is a zoonosis caused by the bacterium Bartonella henselae, a small fastidious gram-negative bacillus that may enter the eye through facial scratches or the conjunctiva.[1] Parinaud oculoglandular syndrome occurs in 5-7% of patients with CSD.[2] [3] Immature domestic cats are the most frequently implicated vector with other sources including dogs, cat fleas, and sandflies.[4][5]
Tularemia
Tularemia is a zoonotic disease caused by the bacterium Francisella tularensis, a pleomorphic aerobic gram-negative coccobacillus. A wide range of animals serve as reservoirs (rabbits, squirrels, game bird, muskrats, hamsters, prairie dogs, sheep, beavers, cats, tick, flea, and mosquito). F. tularensis can be aerosolized and can be found in soil and water.[6]
Sporotrichosis
Sporotrichosis is a rare mycosis caused by Sporothrix schenckii, a dimorphic fungus existing in mycelial and yeast phase. Infection rates are higher in tropical and subtropical areas such as Brazil and Peru,[7] where Sporothrix schenckii is endemic. It is commonly found in plant organic matter such as leaves, wood, soil, and water. [8] Sporothrix schenckii has also been recovered from animal fur, feces, and oral cavities (i.e. cats, horse, dogs, rabbits, and rats).[9]
History
Cat-Scratch Disease
The incubation period is typically anywhere from 3 days to 3 weeks. B. henselae is frequently transmitted through hand-to-eye contact, or from contamination of an open wound.[2] Aerosolized cat flea feces is another proposed mechanism. Unlike other manifestations of CSD, the oculoglandular form rarely manifests after a scratch.[10] A higher number of cases are reported among children and veterinary workers. To evaluate for potential exposure, clinicians should assess for recent contact with cats, dogs, human body lice, sandflies, or fleas. [5] It is important to note that although a history of cat contact is often present, a cat bite or scratch may not be apparent.[1]
Tularemia
Tularemia has an incubation period of approximately 2 to 5 days, and rarely up to 3 weeks. Infected individuals are typically meat-handlers, campers, or hunters who have had recent contact with animals or contaminated particles. The mechanism of ocular involvement is hand-to-eye contact, or splash from contaminated animal body fluids or water.[11] History should include recent travel, outdoor activities, game ingestion, handling of animal carcasses, and tick exposure.
Sporotrichosis
Sporotrichosis can be transmitted through traumatic inoculation of the skin with organic matter,[12] infected respiratory secretions,[7] and hand-to-eye contact with infected animal fluids.[13] Occupations or activities that have been associated with infection include plant or soil handling and contact with sick or healthy animals (i.e. gardener, miners, hunters, farmers, veterinarians, etc).
Physical examination
Cat-Scratch Disease
Ocular signs of Cat-Scratch disease range from scant to copious serous and/or mucoid discharge, mild periorbital edema, conjunctival ulceration overlying a granuloma, and/or conjunctival nodules. Rarely, peripheral corneal ulceration may occur.[1] A papule may appear at the site of inoculation. Patients typically develop tender lymphadenopathy (pre- or post-auricular, submandibular, or cervical lymph nodes). [3][14][15] Less common manifestations are low grade fever, pain, and suppurative lymphadenopathy. It can take several weeks for the granuloma to resolve, and several months for the regional lymphadenopathy to clear.[11]
Tularemia
Oculoglandular syndrome is a rare presentation of Tularemia (2-14%). Signs of ocular involvement include mucopurulent discharge, conjunctival chemosis, corneal epithelial defects, ulceration, nodules, and periorbital edema. Less common ocular signs are dacryocystitis and corneal ulcer. Patients develop headaches, anorexia, high fever, sore throat, and painful lymphadenopathy (preauricular, submandibular, cervical, parotid, or axillary). [10][16]
Sporotrichosis
In Sporotrichosis oculoglandular syndrome, conjunctival granuloma and/or ulcer, eyelid ulcer, and purulent discharge are some common ocular findings.[7][8] Commonly associated signs include non-tender preauricular and submandibular lymphadenopathy. Less commonly, patients experience painful lymphadenopathy, dacryocystitis,[7] or fever.[13]
Diagnosis
Cat-Scratch Disease
Cat-scratch disease is diagnosed with serology, culture,[17] or polymerase chain reaction (PCR).[18] In practice, serology is the most often used modality. Titers of immunoglobulin M (IgM) greater or equal to 1:20, immunoglobulin G (IgG) greater or equal to 1:256, or a fourfold increase in serum IgG titers between acute and convalescent phase are indicative of active infection.[3] If CSD is tested later in the disease course, IgM titers are typically low and IgG titers are high. Therefore, IgG titers of 1:512 are indicative of recent infection. Cultures are difficult to obtain and require long incubation periods. In the past, B. henselae was diagnosed using the Hanger-Rose test on lymph node aspirates.[19] For biopsies, Warthin-Starry, Steiner silver stain, or Brown-Hopp stain can be used to show the presence of gram-negative bacilli. [1]
Tularemia
Tularemia is diagnosed with culture, serology, PCR, or direct fluorescent antibody assay. Potential specimens are lymph node aspirates, conjunctival nodule biopsy, conjunctival swab or serum. In practice, serology is the most often used modality. Serologic titers greater than 1:128 indicate prior or current infection. Because it takes 2 weeks for titers to increase, the first samples should be obtained early, followed by repeat samples at day 14 and weeks 3 to 4. A fourfold increase in F. tularensis antibodies confirms an active infection. Titers greater than 1:160 at 2 to 3 weeks after onset of symptoms onset indicate an active infection.[10] For cultures, the organism needs a cysteine-enriched media to grow—such as modified Thayer-Martin media or chocolate agar (GCII agar with 1% haemoglobin and 1% IsoVitaleX).[20]
Sporotrichosis
Sporotrichosis is typically diagnosed with culture (Sabouraud 2%). If a conjunctival nodule is present, it can be biopsied and sent for histopathology (Gomori-Methenamine-Silver (GMS), Periodic Acid-Schiff (PAS) stains), or electron microscopy (EM).[12]
Management
Cat-Scratch Disease
The role of antibiotic therapy in CSD has not been established in a controlled study. As CSD is generally self-limited, treatment is usually supportive. Broad-spectrum ophthalmic drops are used to prevent secondary infections.[19] Systemic antibiotics are recommended for moderate to severe disease, which more commonly involve immunocompromised patients. A wide variety of antibiotics and antibiotic combinations have been utilized to treat CSD including doxycycline, azithromycin, trimethoprim/sulfamethoxazole DS, ciprofloxacin, and rifampin. The duration of treatment varies depending on the patient’s clinical course, and should be individualized.[3][15]
Tularemia
For severe disease, first line agents are streptomycin or gentamicin for 7 to 14 days. For less severe disease, the recommended treatment is oral or intravenous doxycycline or ciprofloxacin for 14-21 days.[5] Topical treatment options include ciprofloxacin ophthalmic drops, or tobramycin ophthalmic drops or ointment. [11] With therapy, ocular symptoms improve in approximately 1 week, and overall recovery may be seen in 2 to 3 months.[10][21]
Sporotrichosis
There are no controlled studies to evaluate the effectiveness of antifungal agents for the treatment of sporotrichosis. Currently, Sporotrichosis oculoglandular syndrome is treated with oral itraconazole, potassium iodide, or fluconazole. Topical fluconazole ophthalmic drops have also been utilized with success. Nonetheless, sporotrichosis typically responds well to treatment. Duration and treatment are usually weeks to months, and symptoms resolve in 1 to 4 months. [9] [12][13]
References
- ↑ Jump up to: 1.0 1.1 1.2 1.3 1.4 1.5 Mannis MJ, Holland EJ. Cornea. 4th ed. St. Louis, Mo: Elsevier Inc.; 2015:518-525.
- ↑ Jump up to: 2.0 2.1 Carithers HA. Cat-scratch disease. An overview based on a study of 1,200 patients. American journal of diseases of children. 1985;139(11):1124-1133.
- ↑ Jump up to: 3.0 3.1 3.2 3.3 Murakami K, Tsukahara M, Tsuneoka H, et al. Cat scratch disease: analysis of 130 seropositive cases. Journal of infection and chemotherapy: official journal of the Japan Society of Chemotherapy. 2002;8(4):349-352.
- ↑ Cunningham ET, Koehler JE. Ocular bartonellosis. American journal of ophthalmology. 2000;130(3):340-349.
- ↑ Jump up to: 5.0 5.1 5.2 Ormerod LD, Dailey JP. Ocular manifestations of cat-scratch disease. Current opinion in ophthalmology. 1999;10(3):209-216.
- ↑ Nigrovic LE, Wingerter SL. Tularemia. Infectious disease clinics of North America. 2008;22(3):489-504, ix.
- ↑ Jump up to: 7.0 7.1 7.2 7.3 Ramirez Soto MC. Sporotrichosis in the Ocular Adnexa: 21 Cases in an Endemic Area in Peru and Review of the Literature. American journal of ophthalmology. 2016;162:173-179 e173.
- ↑ Jump up to: 8.0 8.1 Lopes-Bezerra LM, Schubach A, Costa RO. Sporothrix schenckii and sporotrichosis. Anais da Academia Brasileira de Ciencias. 2006;78(2):293-308.
- ↑ Jump up to: 9.0 9.1 Gordon DM. Ocular sporotrichosis; report of a case. Archives of ophthalmology. 1947;37(1):56-72.
- ↑ Jump up to: 10.0 10.1 10.2 10.3 Carithers HA. Oculoglandular disease of parinaud. A manifestation of cat-scratch disease. American journal of diseases of children. 1978;132(12):1195-1200.
- ↑ Jump up to: 11.0 11.1 11.2 Eren Gok S, Kocagul Celikbas A, Baykam N, et al. Evaluation of tularemia cases focusing on the oculoglandular form. Journal of infection in developing countries. 2014;8(10):1277-1284.
- ↑ Jump up to: 12.0 12.1 12.2 Hampton DE, Adesina A, Chodosh J. Conjunctival sporotrichosis in the absence of antecedent trauma. Cornea. 2002;21(8):831-833.
- ↑ Jump up to: 13.0 13.1 13.2 Schubach A, de Lima Barros MB, Schubach TM, et al. Primary conjunctival sporotrichosis: two cases from a zoonotic epidemic in Rio de Janeiro, Brazil. Cornea. 2005;24(4):491-493.
- ↑ Jawad AS, Amen AA. Cat-scratch disease presenting as the oculoglandular syndrome of Parinaud: a report of two cases. Postgraduate medical journal. 1990;66(776):467-468.
- ↑ Jump up to: 15.0 15.1 Galindo-Bocero J, Sanchez-Garcia S, Alvarez-Coronado M, Rozas-Reyes P. Parinaud's oculoglandular syndrome: A case report. Archivos de la Sociedad Espanola de Oftalmologia. 2017;92(1):37-39.
- ↑ Raja H, Starr MR, Bakri SJ. Ocular manifestations of tick-borne diseases. Survey of ophthalmology. 2016;61(6):726-744.
- ↑ Grando D, Sullivan LJ, Flexman JP, Watson MW, Andrew JH. Bartonella henselae associated with Parinaud's oculoglandular syndrome. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 1999;28(5):1156-1158.
- ↑ Le HH, Palay DA, Anderson B, Steinberg JP. Conjunctival swab to diagnose ocular cat scratch disease. American journal of ophthalmology. 1994;118(2):249-250.
- ↑ Jump up to: 19.0 19.1 Huang MC, Dreyer E. Parinaud's oculoglandular conjunctivitis and cat-scratch disease. International ophthalmology clinics. 1996;36(3):29-36.
- ↑ Kantardjiev T, Padeshki P, Ivanov IN. Diagnostic approaches for oculoglandular tularemia: advantages of PCR. The British journal of ophthalmology. 2007;91(9):1206-1208.
- ↑ Thompson S, Omphroy L, Oetting T. Parinaud's oculoglandular syndrome attributable to an encounter with a wild rabbit. American journal of ophthalmology. 2001;131(2):283-284.