Ocular Manifestations of Bosma Arrhinia Microphthalmia Syndrome (BAMS)
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
Bosma Arrhinia Microphthalmia Syndrome (BAMS) is a rare condition characterized by nasal and orbital abnormalities in conjunction with endocrine dysfunction. Ocular manifestations include coloboma (iris, choroid, retina, eyelid), cataract formation, anophthalmia or microphthalmia, and nasolacrimal duct obstruction.
Disease Entity
Bosma Arrhinia Microphthalmia Syndrome (BAMS) is a rare condition characterized by nasal and orbital abnormalities in conjunction with endocrine dysfunction. Although the exact pathophysiology is unknown, current research indicates that the Structural Maintenance of Chromosomes Flexible Hinge Domain (SMCHD1), a gene with known silencing functions through epigenetic repression, is most likely implicated in the disrupted embryogenesis in this condition. Patients typically present at birth with a combination of arrhinia (congenital partial or complete absence of the nose) with ocular abnormalities such as microphthalmia, coloboma and/or early cataract formation, and nasolacrimal duct dysgenesis.
History
BAMS is a rare disease first reported by Gifford et al. in 1972, when he described two unrelated patients with congenital arrhinia with eye defects.[1] A report by Bosma in 1981 formally described the condition and noted these patients typically had concurrent reproductive system dysfunction and normal intelligence.[2] Since his report, fewer than 100 cases have been reported worldwide.[3] The underlying cause remained unknown until Shaw conducted a whole exome sequencing project in 2017 involving 40 individuals with arrhinia and 55 of their family members. This study identified the SMCHD1 gene as the most likely underlying mutation contributing to BAMS, however the mechanism through which this mutation leads to the phenotypic presentation has not been elucidated.[4]
Epidemiology
The exact incidence of BAMS is unknown due to its rare occurrence and most information about the condition has been through case reports or small case series. Additionally, congenital arrhinia is seldom reported without associated holoprosencephaly, and fewer than 100 cases have been documented to date. A study by Thiele et al. in 1996 described a three-generation family with BAMS, however most cases are thought to be de novo in origin.[5] To date, no specific risk factors have been identified for this condition, but a recent review of the literature suggests a male to female ratio of 10:4.[6]
Risk Factors
There are currently no known risk factors.
Anatomy
Anatomically, BAMS is characterized by a range of ocular abnormalities that may vary significantly between individuals, ranging from normal eye development to anophthalmia.[6] Diagnostic imaging such as CT or MRI may reveal features like asymmetric microphthalmia or coloboma, which may be evident in the iris, retina, choroid, or optic disc. Abnormalities in the lacrimal structures are also common features of BAMS, often leading to symptoms like epiphora or dacryocystitis.
Craniofacial imaging in BAMS typically reveals a complete absence of nasal bones and septal structures, along with a blind, rudimentary nasopharynx. CT scans often highlight the absent or abnormal sinus structures, while an MRI can highlight the absence of the cribriform plate and olfactory structures. [7] Additional craniofacial abnormalities may include a high-arched palate and a hypoplastic maxilla.[6]
Genetics
Current research on the cause of BAMS indicates that variants of the Structural Maintenance of Chromosomes Flexible Hinge Domain (SMCHD1) gene are implicated in pathogenesis.[4] While the exact function of the SMCHD1 protein is under investigation, it has known silencing functions. It is hypothesized that mutations may cause abnormal silencing of the genes involved in embryogenesis of the head and face, resulting in the characteristic features of the condition. Disease inheritance for BAMS has been found to be passed in an autosomal dominant pattern, however most known cases are de novo.[8]
Embryology
A mutation in the SMCHD1 gene is postulated to silence genes involved in the embryogenesis of the head and face. Particularly, in the eye, the optic vesicle contacts the surface ectoderm at approximately 26-27 days. Eye development occurs between 34-44 days, and closure of anterior and posterior choroidal fissures occurs at 36 and 44 days, respectively. Any insult through this period period may lead to abnormal prenatal ocular development.
| Week 3 | The optic grooves begin to form. |
|---|---|
| Week 4 | The optic vesicle contacts the surface ectoderm causing it to invaginate and create the optic cup. |
| Week 5 | The edges of the optic fissure fuse. |
| Week 6-7 | Development begins for the:
Additionally, the optic fissure completes fusion, the lens separates from the surface ectoderm, and the aqueous chamber forms. |
| Week 9 | The hyaloid vessels fuse with optic stalk.
The ciliary bodies continue to develop and extends to the inner iris. |
| Week 22 | The veins and arteries of the choroid begin to resemble its final form. |
| Month 4 | The cones differentiate and the fovea develops further. |
| Month 5-7 | The upper and lower eyelids separate.
The eyelashes and glands develop. |
Pathophysiology
Leading theories suggest that mutations in the SMCHD1 gene play a key role in the development of BAMS. This gene codes for a silencing protein, though its specific role in the pathogenesis of the condition remains unclear.[4] The downstream effects of SMCHD1 mutations most likely lead to disrupted craniofacial and pituitary development. Another theory proposes that these mutations result in the production of an abnormal protein, DUX4, which may be toxic to nasal precursor cells.[12]
Additionally, abnormal pituitary development associated with BAMS can cause genital dysgenesis, delayed puberty, and skeletal abnormalities secondary to inadequate sex hormone production. Disruption of this embryonic axis results in abnormal olfaction and nasal development.
Diagnosis
Systemic Manifestations
The diagnosis of BAMS is primarily clinical and relies on the characteristic physical features of the condition. Non-ocular manifestations include:
- Congenital arrhinia
- Midface craniofacial hypoplasia
- Hypogonadotropic hypogonadism (undescended testes in males, absence of secondary sex characteristics in females)
- Osteopenia or osteoporosis
Extraocular imaging with DEXA scan may also be employed in patients with hypopituitarism to evaluate bone density. It is important to note that intellectual development is normal.
Ocular Manifestations
- Coloboma of the lens, iris, retina, or choroid
- Microphthalmia: Unilateral or bilateral non-functional, disorganized eye, characterized by an axial length of two standard deviations below the mean for age[13]
- Anophthalmia: Unilateral or bilateral absence of ocular tissue. Distinguishing severe microphthalmia from anophthalmia may be clinically difficult, but may be determined using an axial fat-suppressed T2-weighted MRI[7]
- Visually significant cataract
Ocular Signs
- Microphthalmia or Anophthalmia
- Low vision or blindness
- Angle closure glaucoma
- Retinal detachment
- Refractive error
- Coloboma
- Cataracts
- Retinal detachment
- Glaucoma
- Choroidal neovascularization
- Refractive error
- Nasolacrimal Duct Stenosis
- Recurrent conjunctivitis
- Dacryocystitis
- Epiphora
- Blurred vision
- Preseptal cellulitis
- Conjunctival or eyelid erythema
- Chemosis
Ocular Symptoms
- Light Sensitivity
- Epiphora
- Itching
- Foreign body sensation
- Blurry vision
- Pain
Slit Lamp Examination
- Complete or partial loss of nasal structure
- Microphthalmia
- Anophthalmia
- Cataract
- Pseudopolycoria
- Coloboma (Iris, Lens, Choroid, Retina, Eyelid)
- Punctate Epithelial Erosions or Superficial Punctate Keratitis
- Band Keratopathy
Diagnostic procedures
Imaging studies such as CT and MRI may be used to further evaluate the extent of craniofacial abnormalities and assess for the presence or absence of the pituitary gland.
Computed Tomography
- Advantage:
- Rapid testing, excellent first line test in adults
- Good visualization of bony structures[17]
- Disadvantage:
- Radiation exposure (consider avoiding in pediatric patients)
Magnetic Resonance Imaging
- Advantage:
- Provides more detailed information about the optic tracts, intra-orbital contents, and concurrent intracranial malformations when compared to CT scan[7]
- Reduced radiation exposure
- As BAMS patients require head imaging to manage concurrent conditions throughout their lives, so MRI mitigates lifetime radiation exposure.
- Disadvantage:
- Prolonged duration for imaging
- May require sedation in patients, particularly pediatric patients.
Ultrasound and optical coherence tomography (OCT) are used to detect the presence of a posterior coloboma, while follow-up imaging with MRI is employed to assess the extent of the coloboma.[18]
In addition to physical exam findings and imaging, genetic testing can be employed to confirm mutations in the SMCHD1 gene.
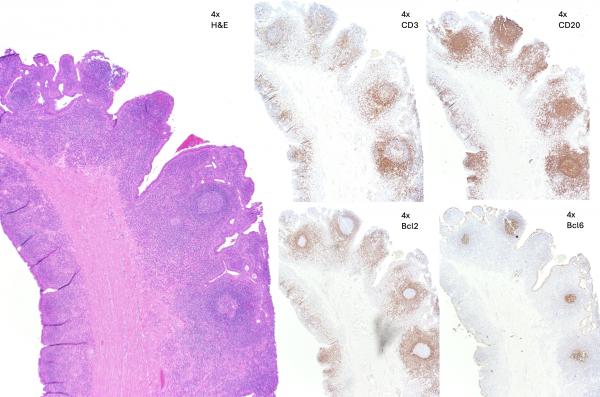
Histopathology
Histopathology related to BAMS in the current literature is associated with specimen from a dacryorhinocystostomy (DCR) procedure. Predominant findings include scar tissue (dense stroma) with chronic lymphocytic infiltrate secondary to nasolacrimal duct obstruction and dacryocystitis.
Differential diagnosis
- CHARGE Syndrome: Coloboma, atresia of the choanae, growth delay, midline craniofacial defects and microphthalmia
- Holoproscencephaly: Cyclopia, microcephaly
- Nager Syndrome: Craniofacial dysostosis, microphthalmia
- Treacher Collins: Coloboma, narrowing of the tear ducts, mandible dysgenesis
- Kallman: Anosmia, hypogonadotropic hypogonadism
Management
Management of BAMS requires a multidisciplinary team including a primary care provider, endocrinology, ophthalmology, otolaryngology, and plastic surgery in addition to social services. Management is tailored to address the patient’s symptoms associated with their phenotypic characteristics. Since disease management guidelines have not been outlined to date, a comprehensive evaluation of ocular, endocrine, and skeletal systems should first be conducted to determine the extent of the disease.
With regards to ocular management, patients may have any combination of a cataract, coloboma, nasolacrimal duct stenosis, microphthalmia or anophthalmia. Early, comprehensive eye exams are essential to first identify these conditions and initiate appropriate management. Serial imaging studies should be considered depending on the associated condition and severity. If the patient has significant unilateral visual deficits secondary to BAMS, they should always be counseled on the use of protective eyewear to preserve vision in the seeing eye.[19] Low vision devices may also be employed as needed and referral to ocular occupational health services may benefit patients.
Microphthalmia and Anophthalmia
The paucity of ocular tissue in the eye socket affects the development of the bony orbit as a child develops. This can lead to asymmetric facial structure in unilateral microphthalmia/anophthalmia and can limit ocular prothesis fitting in the future, without intervention.
Medical
- Monocular precautions with full-time polycarbonate wear
- Routine ocular examination
Non-surgical
- Socket expanders can be used for optimizing facial/orbital development.
- Hydrophillic expanders can be utilized to allow for gradual expansion after initial placement[20]
Surgical
- Orbital Implantation can be performed after at least two-months of socket expansion.
- Implant material can be autologous (dermis fat graft) or man-made (non-porous, or porous) materials.
- Dermis fat graft can grow progressively and can reduce additional surgeries.
- Man-made materials will need additional implant exchange as a child grows.
Coloboma
Coloboma of the iris, retina, and choroid may be classified as either “typical” if they are in the inferior/infero-nasal quadrant or “atypical” if located elsewhere.[14] Coloboma of the eyelid are classified as “typical” in the superomedial quadrant and “atypical” in all other quadrants.
Iris
- Signs and Symptoms
- Iris coloboma are found infero-nasally and may present as a keyhole shape. Depending on the extent of the coloboma, the lack of iris tissue and pseudo-polycoria may impair normal pupillary contraction and cause increased light sensitivity. Patients may have low vision, blindness, or reduced peripheral vision.
- Management
- Corrective lenses should be utilized for any refractive error and contact lenses can be offered for improved cosmetic appearance. In the case of unilateral coloboma, patching may be done to prevent the development of amblyopia. Low vision aids may be considered if the coloboma results in permanent vision loss.[21] Iris coloboma are often associated with lens or zonular coloboma and may be difficult to manage surgically. Surgery can be performed to help the iris constrict in a more circumferential manner.[22]
Chorioretinal
- Signs and Symptoms
- Chorioretinal coloboma are characterized by defects in the retinal or choroidal tissue and are typically located in the inferotemporal quadrant. If it involves the retina, then it will present as an area of whitening on the fundus.[23] Patients are at a higher risk for decreased visual acuity if the coloboma involves the macula or optic disc.
- Management
- One major concern is the increased risk of retinal detachment in this population, which should be monitored with a dilated fundus exam every 6-12 months. The risk of rhegmatogenous retinal detachment has been reported to be up to 40%, but studies indicate that prophylactic laser photocoagulation can decrease this risk significantly.[24]
Eyelid
- Signs and Symptoms
- If large, coloboma of the eyelid may be obvious at birth and place the patient at increased risk of exposure keratopathy.
- Management
- This may be conservatively managed with topical lubrication, protective lenses, and moisture chambers. Intervention by oculoplastics may be considered if symptoms are severe or if there is a desire to improve cosmesis.
Lens
- Signs and Symptoms
- Coloboma of the lens is characterized by absence of a portion of the lens and this defect also includes the section of zonular fibers.
- Management
- Corrective lenses should be given to patients with refractive errors. In the case that the coloboma cannot be managed with lenses alone or if it causes media opacities, the lens may be surgically removed.[14] Presence of a visually significant cataract is an indication for removal with or without IOL placement to avoid development of amblyopia.
- Due to its associated zonular fiber involvement, additional precautions for zonular dehiscence should be made prior to surgical intervention.
Nasolacrimal duct obstruction (NLDO)
Nasolacrimal duct obstruction (NLDO) can involve one or both eyes. It typically presents with excess tearing due to poor drainage through the lacrimal system. Partial or complete obstruction can also cause stagnant fluid in the lacrimal sac and lead to a secondary infection (dacryocystitis). NLDO may resemble chronic infectious conjunctivitis but will not resolve with use of antibiotics. NLDO may be diagnosed with the fluorescein dye disappearance test, where a drop of dye is placed in each eye and monitored over five minutes.[25] Disappearance of the dye indicates patency of the nasolacrimal duct system and can more likely be managed with conservative treatment, while pooling of the dye is more indicative of stenosis.
- Initial conservative management with nasolacrimal massage should be trialed along with lubricating drops and as needed antibiotics for infection.[25]
- Persistence of symptoms past 6 to 12 months is an indication for surgical probing with possible stent placement. In patients with BAMS, concurrent arrhinia and midface hypoplasia may distort normal anatomy and necessitate a more invasive surgical approach such as a DCR with Jones Glass Tube placement.[26]
Prognosis
Newborns are obligate nose breathers and may require respiratory support early in life. They often need the assistance of feeding tubes to gain weight and may have failure to thrive if they are unable to meet their caloric needs. Overall, patients who receive adequate support during their early life typically have normal neurodevelopment and average life spans.[3]
Additional Resources
- Rare Diseases
- Provides information to clinical trials and a contact for Dr. Shaw.
- NIH clinical trials page
- Resource connects the closest trial for a specific condition based on location.
References
- ↑ Gifford, G. H., Jr., Swanson, L., & MacCollum, D. W. (1972). Congenital absence of the nose and anterior nasopharynx: Report of two cases. Plastic and Reconstructive Surgery, 50, 5-12.
- ↑ Bosma, J. F., Henkin, R. I., Christiansen, R. L., & Herdt, J. R. (1981). Hypoplasia of the nose and eyes, hyposmia, hypogeusia, and hypogonadotropic hypogonadism in two males. Journal of Craniofacial Genetics and Developmental Biology, 1, 153-184.
- ↑ 3.0 3.1 National Organization for Rare Disorders. (2024, May 20). Bosma Arrhinia microphthalmia syndrome - symptoms, causes, treatment. National Organization for Rare Disorders. https://rarediseases.org/rare-diseases/bosma-Arrhinia-microphthalmia-syndrome/
- ↑ 4.0 4.1 4.2 Shaw, N. D., Brand, H., Kupchinsky, Z. A., Bengani, H., Plummer, L., Jones, T. I., ... Talkowski, M. E. (2017). SMCHD1 mutations associated with a rare muscular dystrophy can also cause isolated Arrhinia and Bosma Arrhinia microphthalmia syndrome. Nature Genetics, 49(2), 238-248. https://doi.org/10.1038/ng.3743
- ↑ Thiele, H., Musil, A., Nagel, F., & Majewski, F. (1996). Familial Arrhinia, choanal atresia, and microphthalmia. American Journal of Medical Genetics, 63, 310-313.
- ↑ 6.0 6.1 6.2 Brasseur, B., Martin, C. M., Cayci, Z., Burmeister, L., & Schimmenti, L. A. (2016). Bosma Arrhinia microphthalmia syndrome: Clinical report and review of the literature. American Journal of Medical Genetics Part A, 170(5), 1302-1307. https://doi.org/10.1002/ajmg.a.37572
- ↑ 7.0 7.1 7.2 Celebi, A. R., & Sasani, H. (2014). Differentiation of true anophthalmia from clinical anophthalmia using neuroradiological imaging. World Journal of Radiology, 6(7), 515-518. https://doi.org/10.4329/wjr.v6.i7.515
- ↑ Gordon, C. T., Xue, S., Yigit, G., Filali, H., Chen, K., Rosin, N., ... Reversade, B. (2017). De novo mutations in SMCHD1 cause Bosma Arrhinia microphthalmia syndrome and abrogate nasal development. Nature Genetics, 49(2), 249-255. https://doi.org/10.1038/ng.3765
- ↑ Azzam D, Bordoni B. Embryology, Optic Fissure. [Updated 2023 Apr 3]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK554433/
- ↑ Bales TR, Lopez MJ, Clark J. Embryology, Eye. [Updated 2023 Mar 27]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK538480/
- ↑ Rubinstein, T. J., Weber, A. C., & Traboulsi, E. I. (2016). Molecular biology and genetics of embryonic eyelid development. Ophthalmic genetics, 37(3), 252–259. https://doi.org/10.3109/13816810.2015.1071409
- ↑ Inoue, K., Bostan, H., Browne, M. R., et al. (2023). DUX4 double whammy: The transcription factor that causes a rare muscular dystrophy also kills the precursors of the human nose. Science Advances, 9(7), eabq7744. https://doi.org/10.1126/sciadv.abq7744
- ↑ 13.0 13.1 Ragge, N. K., Subak-Sharpe, I. D., & Collin, J. R. (2007). A practical guide to the management of anophthalmia and microphthalmia. Eye (London, England), 21(10), 1290-1300. https://doi.org/10.1038/sj.eye.6702858
- ↑ 14.0 14.1 14.2 Lingam, G., Sen, A. C., Lingam, V., Bhende, M., Padhi, T. R., & Xinyi, S. (2021). Ocular coloboma: A comprehensive review for the clinician. Eye (London, England), 35(8), 2086-2109. https://doi.org/10.1038/s41433-021-01501-5
- ↑ StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK532873/
- ↑ Pezzoli M, Zeppieri M, Patel BC. Dacryostenosis. [Updated 2024 Mar 6]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK563132/
- ↑ Graham, J. M., Jr., & Lee, J. (2006). Bosma Arrhinia microphthalmia syndrome. American Journal of Medical Genetics Part A, 140(2), 189-193. https://doi.org/10.1002/ajmg.a.31039
- ↑ Vegunta, S., & Patel, B. C. (2024). Optic nerve coloboma. In StatPearls [Internet]. StatPearls Publishing. https://www.ncbi.nlm.nih.gov/books/NBK532877/
- ↑ Mahan, M., & Purt, B. (2024). Ocular trauma prevention strategies and patient counseling. In StatPearls [Internet]. StatPearls Publishing. https://www.ncbi.nlm.nih.gov/books/NBK580537/
- ↑ Alanazi, R. R., Schellini, S. A., Alhussain, H., Elkhamary, S., Khandekar, R., & AlSheikh, O. (2022). Outcomes of the use of orbital hydrogel expanders in the management of congenital anophthalmia: CT-based orbital parameter analysis. Orbit (Amsterdam, Netherlands), 41(6), 691-699. https://doi.org/10.1080/01676830.2021.1990350
- ↑ Porter, D. (2024) What is a Coloboma? American Academy of Ophthalmology. https://www.aao.org/eye-health/diseases/what-is-coloboma
- ↑ Cionni, R., Karatza, E., Osher, R., & Shah, M. (2006). Surgical technique for iris coloboma repair. Journal of Cataract & Refractive Surgery, 32(11), 1913-1916.
- ↑ Uhumwangho, O. M., & Jalali, S. (2014). Chorioretinal coloboma in a paediatric population. Eye (London, England), 28(6), 728-733. https://doi.org/10.1038/eye.2014.61
- ↑ Hussain, R. M., Abbey, A. M., Shah, A. R., Drenser, K. A., Trese, M. T., & Capone, A., Jr. (2017). Chorioretinal coloboma complications: Retinal detachment and choroidal neovascular membrane. Journal of Ophthalmic & Vision Research, 12(1), 3-10. https://doi.org/10.4103/2008-322X.200163
- ↑ 25.0 25.1 Olitsky, S. E. (2014). Update on congenital nasolacrimal duct obstruction. International Ophthalmology Clinics, 54(3), 1-7. https://doi.org/10.1097/IIO.0000000000000030
- ↑ Jones, D. C. R., Eisenbach, N., Karni, O., Sela, E., Nemet, A., Dror, A., Levy, E., Kassif, Y., Ovadya, R., Ronen, O., & Marshak, T. (2021). Conjunctivodacryocystorhinostomy (CDCR) success rates and complications in endoscopic vs non-endoscopic approaches: A systematic review. International Forum of Allergy & Rhinology, 11(2), 174-194.

