Neuro-ophthalmic Findings in Coma
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
Ophthalmologists and neurologists are often required to evaluate patients in varying degrees of consciousness. This article focuses on the ocular findings present in comatose patients that can be used to localize deficits, diagnose pathology, and prognosticate for possible recovery.
Indications
Coma is a state of prolonged unconsciousness in which a person is alive but unresponsive to all external stimuli, noxious or otherwise.[1] Coma is typically caused by significant injury to the brain. The many etiologies of a comatose state include trauma, stroke, brain tumors, severe hyper- or hypoglycemia, prolonged cerebral hypoxia, seizures, and toxins.[2] While individuals in such a state have lost their ability to think and lost their awareness of their surroundings, they retain non-cognitive functions and a normal sleep pattern.[3] Many people gradually recover after coma but others may enter a persistent vegetative state or die. The outcome largely depends on the cause, severity, and site of the neurological damage. Those who survive may emerge with lasting physical, intellectual, and physiological deficits that require special care.
Coma is a medical emergency that requires timely and appropriate action to preserve life and brain function. This EyeWiki describes the neuro-ophthalmic findings in coma including lid, pupil, ocular motility, and ophthalmoscopic findings.
Exam
The primary goals of the neuro-ophthalmic exam are to help determine the etiology, localization, and severity of the coma.
The severity of a coma can be assessed with the Glasgow Coma Scale (GCS). GCS assesses eye-opening response, verbal response, and motor response and classifies patients with either severe, moderate, or mild brain injury. A patient with a GCS score below 8 is generally considered to be in a coma. The exam is sometimes limited by factors such as drug use, alcohol intoxication, and shock.[4] Moreover, the GCS does not include an examination of eye movements. The Full Outline of Unresponsiveness (FOUR) score is another assessment that includes eye response, motor response, brainstem reflexes, and respiration. Both GCS and FOUR are excellent predictors of final outcome. [5]
Brainstem function should also be assessed if the patient is in a coma. Depending on the level of brainstem dysfunction, respiration patterns such as Cheyne-Stokes respiration, Kussmaul’s breathing, and central neurogenic hyperventilation can be observed. Cranial nerve assessment (including ophthalmoplegia, anisocoria, and ptosis) is also helpful for localizing brainstem lesions. Pupillary responsiveness to light is one of the predictors of poor outcome in TBI. In addition, vital signs such as bradycardia and widened pulse pressure in addition to papilledema or sixth nerve palsy can be indicative of increased intracranial pressure, contributing to brain herniation.[4] Focal findings can be suggestive of a structural lesion (e.g., hemiparesis, aphasia, conjugate eye deviation, and unilateral hypo- or hyperreflexia).[4]
Limitations
Despite its clinical localizing value, the neuro-ophthalmic exam on comatose patients has certain limitations. Sedation, often given to facilitate intubation, can dampen neurological responses and thus lead to exam findings that are mis-attributed to brain damage. An important exception is the evaluation of pupil size which remains a highly sensitive test despite sedation.[6] Furthermore, early on in emergency situations, healthcare workers may not have a complete medical history of the comatose patient. This can cause abnormalities in the ocular exam to be attributed to acute brain damage when in fact they are due to preexisting neurologic or metabolic disorders or pharmacologic agents such as opioids. This can lead to a misdiagnosis and subsequent inappropriate management of the patient.[6] Additionally, inter-observer variability in the ocular evaluation of comatose patients has been observed in multiple studies.[6][7][8] Finally, some of the individual components of the exam have their own limitations. Testing for the oculocephalic reflex requires a stable spine while caloric testing requires an intact eardrum.
Neuro-ophthalmic findings in coma
Spontaneous eye movements
Voluntary eye movements are tested by passively opening the eyes of the patient and observing for any purposeful eye movements. Any purposeful eye movement in an otherwise unresponsive patient should be checked for catatonia, locked-in syndrome, or a feigned coma. If a complete lack of voluntary eye movement response is noted then brainstem involvement should be evaluated using the doll’s head maneuver.[4] Activation of the vestibulo-ocular reflex (VOR) with passive rotation of the head tests the integrity of the brainstem nuclei and infranuclear ocular motor pathways. An intact VOR (normal doll’s head maneuver) suggests a supranuclear cause for the coma.
- Roving eye movements are typically slow, horizontal, bilateral conjugate deviations that are normally seen in light sleep. In comatose patients, the presence of these eye movements suggests a supranuclear (i.e., cortical) etiology (e.g., toxic-metabolic or other bilateral hemispheric etiologies).[4]
- Ocular bobbing is a conjugate, rapid, bilateral, simultaneous downward jerk (i.e., like a fishing bob) of the eyes with a slow return to mid-position suggestive of an acute pontine lesion.[4]
- Inverse ocular bobbing (aka ocular dipping) is an abnormal spontaneous eye movement that consists of a slow downward eye movement followed by a delayed quick return upward to mid-position. This finding is most commonly seen in individuals with a destructive lesion of the pons. It has also been observed in cases of cerebellar hemorrhage, obstructive hydrocephalus, and metabolic encephalopathy.[9]
- Reverse bobbing, a major variant of ocular bobbing, consists of a quick upward eye movement followed by a slow downward return to mid-position and is most commonly seen with metabolic encephalopathy.[9] It has also been seen with combined phenothiazine and benzodiazepine poisoning.[10] Less common variants of this movement have also been reported in the literature.
Visual Pursuit
Visual pursuit can be tested with an object, preferably a mirror, in front of the patient’s eyes. The object is moved in the horizontal and vertical plane to evoke evidence of visual tracking. Smooth visual pursuit of a moving object requires attention and voluntary behaviour; therefore, evidence of smooth visual pursuit suggests a minimally conscious state rather than a vegetative state. Cortical blindness (afferent visual loss) can produce impaired voluntary pursuit but should not affect the movement of eyes to command or to VOR stimulation (i.e., doll’s head maneuver).[11][12]
Blink-to-threat
Blink-to-threat is performed with rapid hand movement toward the patient’s eyes from different directions to elicit a blink reflex. This test may have some utility in comatose patients to assess the presence of afferent input (i.e., intact vision).[11][13]
Pupillary response
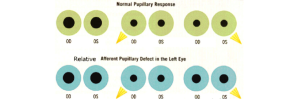
The pupillary light reflex is easily tested and can help with localizing the cause of the coma. To perform this test, first, observe the pupil size in ambient and dim lighting for anisocoria. Shine a bright light into one eye and then alternate between each eye, observing both pupils for any reactivity. Note if the pupils react briskly, sluggishly, or have no response. Swinging the flash light from one eye to the other can detect a relative afferent pupillary defect.
Findings
A normal pupil examination includes equal sized (i.e., isocoric) pupils in light and dark illumination; normal direct and consensual pupillary constriction to light stimulus; round and regular pupils; an intact near stimulus (requires voluntary effort), and the absence of a RAPD.
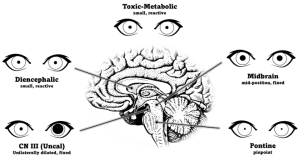
- Unilateral dilated and unreactive pupil
- A unilateral dilated, fixed, and nonreactive pupil in a comatose patient can be an urgent finding (i.e., Hutchinson pupil) from compression of the third nerve at the Kernohan notch due to uncal herniation. Any compressive lesion along the third nerve (e.g., ruptured posterior communicating artery aneurysm) can cause coma. Inadvertent pharmacologic dilation (e.g., atropine drops, scopolamine patch, atropine based inhalers) can mimic the Hutchinson pupil. Administration of topical 1% pilocarpine will not constrict an atropinized, pharmacologically dilated pupil and can be used as a pharmacologic test to differentiate from third nerve palsy.[15]
- Bilateral pinpoint pupils
- Typically dorsal pontine injury
- Injuries such as pontine hemorrhage can disrupt the pupillodilator pathway causing near-maximal constriction[15]
- Bilateral, small, but reactive pupils
- a nonspecific finding
- a very resistant response in the absence of structural damage to the brain and can be suggestive of metabolic or systemic pharmacologic etiologies
- injury to the diencephalon and many toxins and drugs have a similar effect[15]
- Unilateral, small, but reactive pupil
- Horner syndrome can be localized to damage to the ipsilateral oculosympathic pathway (hypothalamus to posterolateral brainstem (first order neuron) descending caudally to ciliospinal center of Budge (cervical C8 to thoracic T2 level in spinal cord), ascending the sympathetic chain to the internal carotid artery then the cavernous sinus, cranial nerve VI then CN V1 to the eye. In addition to the miosis, anisocoria worse in the dark, the presence of ipsilateral ptosis, upside down ptosis, and anhidrosis supports the diagnosis of Horner syndrome. Topical confirmation with cocaine or apraclonidine drops might confirm the Horner syndrome.[15]
- Bilateral fixed and dilated pupils
- suggests CN III dysfunction as seen in bilateral infarction of the dorsal midbrain
- the size of the pupils can be either large or mid-position depending on the preservation of sympathetic tracts[15]
Extraocular motility and ocular alignment
- One or both eyes may be turned inward (i.e., esotropia), turned outward (i.e., exotropia) or turned upward (i.e., hypertropia) or downward (i.e., hypotropia). Ocular motor cranial neuropathy (CN III, IV, VI) or skew deviation may be present in comatose patients. In CN III palsy the eye is typically down and out (hypotropia and exotropia) and CN VI palsy the eye is typically deviated inward (esotropia).
- A lesion involving the frontal eye fields results in conjugate deviation towards the side of the lesion.
- A lesion involving the pons, specifically the paramedian pontine reticular formation and/or abducens nucleus, causes conjugate deviation away from the side of the lesion.[11]
- A saccade is a rapid conjugate movement of the eyes in order to correct our gaze between fixation points. They are performed both voluntarily and involuntarily, such as during reading or rapid eye movement sleep, respectively. For horizontal saccades, pathways in the brain include the frontal eye fields, pons, CN VI, CN III, and the medial longitudinal fasciculus. Deficits in any of these areas can cause abnormal saccades (e.g. ipsilateral saccade).[16]Preserved saccades in a comatose patient is suggestive of a feigned coma or some level of consciousness.[17]
- Smooth visual pursuit describes a slow, tracking movement of the eyes. It is a fully voluntary movement designed for tracking a moving stimulus and maintaining its focus on the fovea. The voluntary nature of smooth visual pursuit indicates that the patient is in at least a minimally conscious state, rather than a vegetative state. Visual pursuit in a comatose patient can be assessed with a mirror test and is described in another section.[17]
- The vestibulo-ocular reflex stabilizes gaze with head turning due to input from the vestibular system. An intact vestibulo-ocular reflex will cause conjugate movement of the eyes opposite of the direction of the head turn, thus maintaining the person’s gaze at a relatively similar spot and preventing images from “slipping”.[17] The methods of testing the vestibulo-ocular reflex are explored in another section.
Vestibulo-ocular Reflex
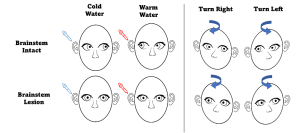
Oculocephalic reflex (Doll’s Eye maneuvers)
Before proceeding with this test, ensure that the patient has a stable cervical spine. During the test, the patient’s eyelids are held open, and the head is briskly rotated from side to side. It is important to rotate fast enough to overcome pursuit. A positive reflex is seen when there is conjugate eye movement in the opposite direction of head rotation, with a gradual return to midline position.[11] This positive finding demonstrates that CN III, VI, VIII, corresponding nuclei in the midbrain/pons, and the vestibular system are intact bilaterally. A negative finding, as seen with eyes in the midline position moving in the same direction as head rotation, similar to a doll’s eyes, suggests brainstem dysfunction from the level of the midbrain to the pons.[18]
Caloric Testing Before proceeding with this test, ensure the patient has an intact tympanic membrane. A small amount of fluid (water or air), either 7oC over (warm) or 7oC under (cold) the bodily temperature, is flushed into one of the ear canals. A positive finding will show conjugate horizontal nystagmus; however, the direction of the nystagmus is dependent on the relative temperature of the fluid. Warm fluid will cause the fast phase of nystagmus to be towards the irrigated ear, while cold fluid will cause the fast phase to be away from the irrigated ear.[19] One study showed that a positive finding in a comatose patient is a strong predictor (PPV=0.93) for emergence from a vegetative state.[20] Another study showed that the absence of a vestibulo-ocular reflex predicted 100% of cerebral death.[21] Similar to the oculocephalic reflex, a negative finding indicates a deep metabolic coma, vestibular damage, or brainstem dysfunction from the level of the midbrain to the pons.
Corneal reflex
When a tactile stimulus, such as a cotton ball, is applied to the surface of the cornea, bilateral blinking indicates the presence of the corneal reflex. A positive corneal reflex in both eyes demonstrates that CN V, VII are intact bilaterally. The absence of a reflex may indicate a lesion of CN V, VII, and/or the pons, but can also be caused by weakness of the orbicularis oculi muscle or ocular pathology.[22]
Fundoscopic exam
The funduscopic exam is performed on non-dilated pupils so that the pupils can be appropriately monitored during coma. Papilledema is suggestive of high intracranial pressure, possibly due to intracranial mass or hemorrhage. Intraocular hemorrhage can be suggestive of Terson syndrome, a finding in which acutely raised intracranial pressure from subarachnoid hemorrhage or traumatic brain injury causes vitreous, subhyaloid, intraretinal, or subretinal bleeding.[23]
Summary
In summary, lid (ptosis), pupil (anisocoria, RAPD, poor light reaction), ocular motility (ocular bobbing, inverse/reverse bobbing, ocular dipping, ophthalmoplegia, ocular misalignment), and ophthalmoscopic (papilledema) findings may help to localize lesions and inform the differential diagnosis in patients with coma.
References
- ↑ Liu G. T. (1999). Coma. Neurosurgery clinics of North America, 10(4), 579–viii.
- ↑ Mayo Foundation for Medical Education and Research. (2020, November 20). Coma. Mayo Clinic. Retrieved March 13, 2022, from https://www.mayoclinic.org/diseases-conditions/coma/symptoms-causes/syc-20371099#:~:text=Coma%20is%20a%20state%20of,preserve%20life%20and%20brain%20function
- ↑ Coma and persistent vegetative state. Cleveland Clinic. (n.d.). Retrieved March 13, 2022, from https://my.clevelandclinic.org/health/articles/6007-coma--persistent-vegetative-state
- ↑ 4.0 4.1 4.2 4.3 4.4 4.5 Bateman DE. NEUROLOGICAL ASSESSMENT OF COMA. Journal of Neurology, Neurosurgery & Psychiatry 2001;71:i13-i17.
- ↑ Nishant Agrawal, Shivakumar S Iyer, Vishwanath Patil, Sampada Kulkarni, Jignesh N Shah, Prashant Jedge.TraumaComparison of admission GCS score to admission GCS-P and FOUR scores for prediction of outcomes among patients with traumatic brain injury in the intensive care unit in India. Acute and Critical Care 2023;38(2):226-233. DOI: https://doi.org/10.4266/acc.2023.00570. Published online: May 25, 2023
- ↑ 6.0 6.1 6.2 Schmidt WU, Lutz M, Ploner CJ, Braun M. The diagnostic value of the neurological examination in coma of unknown etiology. J Neurol. 2021 Apr;268,3826–3834. https://doi.org/10.1007/s00415-021-10527-4
- ↑ Holdgate A, Ching N, Angonese L. Variability in agreement between physicians and nurses when measuring the Glasgow Coma Scale in the emergency department limits its clinical usefulness. Emerg Med Australas. 2006 Aug;18(4):379-84. doi: 10.1111/j.1742-6723.2006.00867.x. PMID: 16842308.
- ↑ Gill MR, Reiley DG, Green SM. Interrater reliability of Glasgow Coma Scale scores in the emergency department. Ann Emerg Med. 2004 Feb;43(2):215-23. doi: 10.1016/s0196-0644(03)00814-x. PMID: 14747811.
- ↑ 9.0 9.1 Titer, E. M., & Laureno, R. (1988). Inverse/reverse ocular bobbing. Annals of neurology, 23(1), 103–104. https://doi.org/10.1002/ana.410230126
- ↑ Lennox G. (1993). Reverse ocular bobbing due to combined phenothiazine and benzodiazepine poisoning. Journal of neurology, neurosurgery, and psychiatry, 56(10), 1136–1137. https://doi.org/10.1136/jnnp.56.10.1136
- ↑ 11.0 11.1 11.2 11.3 Zakaria, Z., Abdullah, M. M., Halim, S. A., Ghani, A., Idris, Z., & Abdullah, J. M. (2020). The Neurological Exam of a Comatose Patient: An Essential Practical Guide. The Malaysian journal of medical sciences : MJMS, 27(5), 108–123. https://doi.org/10.21315/mjms2020.27.5.11
- ↑ Kondziella, D., Bender, A., Diserens, K., van Erp, W., Estraneo, A., Formisano, R., Laureys, S., Naccache, L., Ozturk, S., Rohaut, B., Sitt, J.D., Stender, J., Tiainen, M., Rossetti, A.O., Gosseries, O., Chatelle, C. and (2020), European Academy of Neurology guideline on the diagnosis of coma and other disorders of consciousness. Eur J Neurol, 27: 741-756. https://doi.org/10.1111/ene.14151
- ↑ Liu, G. T., & Ronthal, M. (1992). Reflex blink to visual threat. Journal of clinical neuro-ophthalmology, 12(1), 47–56.
- ↑ Lindfield, D., & Das-Bhaumik, R. (2009, March 26). Emergency department management of penetrating eye injuries. International Emergency Nursing. Retrieved March 23, 2022, from https://www.sciencedirect.com/science/article/pii/S1755599X09000056?via%3Dihub
- ↑ 15.0 15.1 15.2 15.3 15.4 Posner JB, Saper CB, Schiff N, Plum F. Plum and Posner’s diagnosis of stupor and coma. 4th ed. UK: Oxford University Press; 2013.
- ↑ Hunfalvay, M., Roberts, C. M., Murray, N., Tyagi, A., Kelly, H., & Bolte, T. (2019). Horizontal and vertical self-paced saccades as a diagnostic marker of traumatic brain injury. Concussion (London, England), 4(1), CNC60. https://doi.org/10.2217/cnc-2019-0001
- ↑ 17.0 17.1 17.2 Purves D, Augustine GJ, Fitzpatrick D, et al., editors. Neuroscience. 2nd edition. Sunderland (MA): Sinauer Associates; 2001. Types of Eye Movements and Their Functions. Available from: https://www.ncbi.nlm.nih.gov/books/NBK10991/
- ↑ Dishion E, Tadi P. Doll's Eyes. [Updated 2021 May 9]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK551716/
- ↑ Gonçalves DU, Felipe L, Lima TM. Interpretation and use of caloric testing. Braz J Otorhinolaryngol. 2008 May-Jun;74(3):440-6. doi: 10.1016/s1808-8694(15)30580-2. PMID: 18661020.
- ↑ Weiss N, Tadie JM, Faugeras F, Diehl JL, Fagon JY, Guerot E. Can fast-component of nystagmus on caloric vestibulo-ocular responses predict emergence from vegetative state in ICU? J Neurol. 2012 Jan;259(1):70-6. doi: 10.1007/s00415-011-6120-z. Epub 2011 Jun 12. PMID: 21667223.
- ↑ Meneses E, Sampaio A, Venosa A, Tauil P, Dias M, Oliveira C. Vestibulo-ocular reflex as predictor of cerebral death in comatose patients. Int Tinnitus J. 2010;16(1):8-13. PMID: 21609907.
- ↑ Miller NR, Newman NJ, Biousse, V, Kerrison, JB, et al. Walsh and Hoyt’s Clinical Neuro-Ophthalmology Sixth edition. 2005;1(6).
- ↑ Terson, A. (1900). De l'hémorrhagie dans le corps vitre au cours de l'hémorrhagie cerebrale. Clin Ophthalmol, 6, 309-312.