Mucormycosis
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
Disease Entity
Disease
Mucormycosis, a subtype of a larger category of diseases known as zygomycoses, is an aggressive opportunistic infection which tends to show a proclivity for the rhino-orbital tract[2] While rhino-orbital mucormycosis is typically associated with Rhizopus species, a number of other species have also been implicated, including Mucor, Rhizomucor, Absidia, Apophysomyces, Saksenaea, Cunninghamella, Cokeromyces, and Syncephalastrum.[3]
Epidemiology
Rhino-orbital mucormycosis tends to affect specific patient populations. It is most often associated with diabetes mellitus (type 2 more frequently than type 1, especially with a history of diabetic ketoacidosis). Other associated conditions include neutropenia, hematologic malignancy, chronic steroid or immunosuppressive drug use, history of transplant, and history of multiple blood transfusions.[2] [4]
Diagnosis
Signs and Symptoms
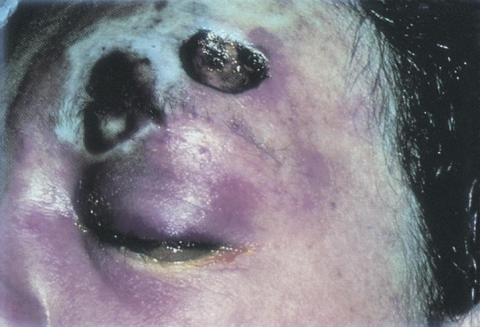
Initial symptoms of sinus-involving mucormycosis are consistent with acute or chronic sinusitis; congestion, blood-tinged secretions, rhinorrhea, headaches, fever, and malaise. Findings of pre-septal cellulitis (soft tissue swelling, eyelid edema/erythema, and conjunctival injection) are also possible. Involvement of the orbit is heralded by findings of chemosis, proptosis, extra-ocular motility deficits, multiple cranial neuropathies (namely CN III, V-1, V-2 and/or VI secondary to cavernous sinus involvement/thrombosis), and loss of vision. Involvement of the contralateral eye can be an indication of extension, namely via the cavernous sinus. Development of neurologic findings such as mental status changes, seizures, or other focal neurologic deficits can indicate intra-cranial extension. [5][6][7]
Diagnostic procedures
In addition to identification of the above clinical signs and symptoms, imaging can be helpful in diagnosis. Contrasted computed tomography (CT) of the orbits and/or paranasal sinuses can show contrast-enhancing hypodense soft-tissue thickening of the involved sinuses, most commonly the ethmoid and maxillary sinuses. Magnetic resonance imaging (MRI) can show T2 iso-intense to hypo-intense soft tissue thickening and heterogenous post-contrast enhancement. Bony erosion is less common but can occur.[8] Diffusion-weighted images can show increased intensity in the affected areas secondary to restricted diffusion from infarcted mucosa.[9] Direct visualization of the nasal mucosa and para-nasal sinuses can reveal dark, necrotic tissue and a characteristic black eschar which results from vascular invasion and tissue infarction.[10]
Definitive diagnosis of mucormycosis can be made via histopathologic evaluation of tissue via biopsy or scraping. Staining of biopsy specimens with calcaflour white, periodic-acid Schiff (PAS), or Grocott-Gomori methenamine-silver stains demonstrates broad, irregular, non-septated hyphae branching at 90 degrees, which are pathognomonic for mucormycosis[11].
Management
General treatment
Early recognition and initiation of appropriate treatment is of paramount importance with regards to outcomes. Urgent correction of underlying metabolic derangements such as hyperglycemia and metabolic ketoacidosis and tapering of corticosteroids or immunosuppressive medications should be pursued if possible. The mainstay of medical treatment remains intravenous anti-fungals, namely lipid-soluble amphoteracin-B at a dose of 5-7.5 mg/kg/day.[12] Although older formulations of azole antifungals (fluconazole, voriconazole) have shown no efficacy in treatment of mucormycosis, recent studies have shown some success with posaconazole as a second line agent or in combination with amphoteracin.[12] Additionally, recent case reports have detailed the use of adjunctive transcutaneous retrobulbar amphotericin B injections (5mL of 1mg/ml daily for 7 days) in additional to intravenous therapy.[13] Recently, studies have shown a correlation of decreased rates of exenteration in patients receiving retrobulbar administration of amphoteracin-B, but without change in mortality rate.[14][15] Finally, hyperbaric oxygen (100% oxygen saturation at 2.5 - 3 ATA) and iron chelation therapy have both been described in the literature as possible adjunctive treatment modalities.[16]
Due to the vascular compromise and tissue necrosis associated with local angio-invasion, intravenous anti-fungal agents tend to have poor tissue penetration. Early surgical debridement of involved tissue, therefore, must be pursued. Evidence suggests that patients who do not undergo early surgical debridement had significantly higher mortality rates than those who did.[17] No clear guideline exists to define the extent of debridement. Extending debridement until freshly bleeding, viable tissue is encountered is a generally accepted principle. Additionally, the use of intra-operative frozen sections to determine if clear margins have been achieved can be useful.[10][18]
Prognosis
Rhino-orbital mucormycosis is a grave disease with significant mortality rates, with some published studies reporting mortality rates of up to 80% or more if untreated.[3] Even with prompt treatment, the prognosis remains guarded. In a series of 929 patients published in 2005, the survival rates for patients treated with amphotericin alone, surgical debridement alone, and both amphotericin and surgical debridement were 61%, 57%, and 70%, respectively.[2] Delay of treatment has been well correlated to poor outcomes, with one study showing a significant difference in survival between patients with a lag time to treatment of 7-12 days (63%) vs patients with lag to treatment time of 13-30 days (44%). Another study reports survival rates of 85% in patients with a lag time of 3-9 days versus just 55% in patients with a lag time of 10-45 days. Development of hemiplegia, facial/eyelid gangrene, and cerebral invasion were all associated with a poor prognosis.[5]
Conclusion
Rhino-orbital mucormycosis is an aggressive invasive fungal infection which tends to affect patients with a history of diabetes (especially with ketoacidosis), chronic steroid use, and immunosuppression. Early recognition of symptoms and prompt initiation of treatment, both with intravenous antifungal agents and aggressive surgical debridement are crucial to improving clinical outcomes, though the prognosis for patients with this disease remains guarded even with rapid recognition and early treatment
References
- ↑ American Academy of Ophthalmology. Mucormycosis, left orbit. https://www.aao.org/image/mucormycosis-left-orbit-2 Accessed July 27, 2020.
- ↑ Jump up to: 2.0 2.1 2.2 Roden MM, Zaoutis TE, Buchanan WL, Knudsen TA, Sarkisova TA, Schaufele RL, Sein M, Sein T, Chiou CC, Chu JH, Kontoyiannis DP, Walsh TJ. Epidemiology and outcome of zygomicosis: a review of 929 reported cases. Clin Infect Dis. 2005;41:634–653
- ↑ Jump up to: 3.0 3.1 Ribes JA, Vanover-sams CL, Baker DJ. Zygomycetes in human disease. Clin Microbiol Rev. 2000;13(2):236-301.
- ↑ Kasapoglu F, Coskun H, Ozmen OA, Akalin H, Ener B. Acute invasive fungal rhinosinusitis: evaluation of 26 patients treated with endonasal or open surgical procedures. Otolaryngol Head Neck Surg. 2010;143(5):614-20.
- ↑ Jump up to: 5.0 5.1 Yohai RA, Bullock JD, Aziz AA, Markert RJ. Survival factors in rhino-orbital-cerebral mucormycosis. Surv Ophthalmol. 1994;39(1):3-22.
- ↑ Karadeniz uğurlu Ş, Selim S, Kopar A, Songu M. Rhino-orbital Mucormycosis: Clinical Findings and Treatment Outcomes of Four Cases. Turk J Ophthalmol. 2015;45(4):169-174
- ↑ Gamaletsou MN, Sipsas NV, Roilides E, Walsh TJ. Rhino-orbital-cerebral mucormycosis. Curr Infect Dis Rep. 2012;14(4):423-34
- ↑ Therakathu, J., Prabhu, S., Irodi, A., Sudhakar, S.V., Yadav, V.K., Rupa, V. Imaging features of rhinocerebral mucormycosis: A study of 43 patients. Egyptian Journal of Radiology and Nuclear Medicine 2018; 49(2) 447-452.
- ↑ Safder S, Carpenter JS, Roberts TD, Bailey N. The "Black Turbinate" sign: An early MR imaging finding of nasal mucormycosis. AJNR Am J Neuroradiol. 2010;31(4):771-4
- ↑ Jump up to: 10.0 10.1 Spellberg B, Edwards J, Ibrahim A. Novel perspectives on mucormycosis: pathophysiology, presentation, and management. Clin Microbiol Rev. 2005;18(3):556-69.
- ↑ Skiada A, Lanternier F, Groll AH, et al. Diagnosis and treatment of mucormycosis in patients with hematological malignancies: guidelines from the 3rd European Conference on Infections in Leukemia (ECIL 3). Haematologica. 2013;98(4):492-504.
- ↑ Jump up to: 12.0 12.1 Vehreschild JJ, Birtel A, Vehreschild MJ, et al. Mucormycosis treated with posaconazole: review of 96 case reports. Crit Rev Microbiol. 2013;39(3):310-24.
- ↑ Hirabayashi, Kristin E. M.D.*; Kalin-Hajdu, Evan M.D.*; Brodie, Frank L. M.D.*; Kersten, Robert C. M.D.*; Russell, Matthew S. M.D.†; Vagefi, M. Reza M.D.*. Retrobulbar Injection of Amphotericin B for Orbital Mucormycosis. Ophthalmic Plastic and Reconstructive Surgery 33(4):p e94-e97, July/August 2017.
- ↑ Joos ZP, Patel BC. Intraorbital Irrigation of Amphotericin B in the Treatment of Rhino-Orbital Mucormycosis. Ophthalmic Plast Reconstr Surg. 2017;33(1):e13-e16.
- ↑ Shakrawal, J., Sharma, V., Goyal, A. et al. Outcomes of transcutaneous retrobulbar Amphotericin B (TRAMB) as an adjuvant therapy for rhino-orbital-cerebral mucormycosis (ROCM) following COVID-19. Int Ophthalmol 43, 1919–1926 (2023).
- ↑ Mohamed MS, Abdel-motaleb HY, Mobarak FA. Management of rhino-orbital mucormycosis. Saudi Med J. 2015;36(7):865-8.
- ↑ Zahoor BA, Piercey JE, Wall DR, Tetsworth KD. A surgical approach in the management of mucormycosis in a trauma patient. Ann R Coll Surg Engl. 2016;98(8):e173-e177
- ↑ Spellberg B, Ibrahim AS. Recent advances in the treatment of mucormycosis. Curr Infect Dis Rep. 2010;12(6):423-9.