Mitomycin Intravascular Chemoembolization (MICE)
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
Background
Mitomycin intravascular chemoembolization (MICE) is a novel surgical technique first described by Dean P. Ouano, MD, in 2022 for the treatment of visually significant corneal neovascularization and lipid keratopathy.[1]
Pathophysiology of corneal neovascularization and lipid keratopathy
Optical clarity and optimal vision depend on the transparency and avascularity of the cornea.[2][3] The avascularity of the cornea is maintained through a balance of pro-angiogenic and anti-angiogenic factors which may become imbalanced resulting in corneal neovascularization and subsequent lipid keratopathy where fat deposits accumulate in the cornea adjacent to these vessels resulting in opacification, irregular astigmatism, and decreased visual acuity.[2][3] There are many potential inciting causes including infectious [trachoma, herpetic (herpes simplex or herpes zoster), bacterial, fungal], traumatic (chemical burns), and iatrogenic (contact lens, corneal transplant, intracorneal ring segments).[2]
Treatment strategies for corneal neovascularization and lipid keratopathy
The primary approach for the treatment of corneal neovascularization and lipid keratopathy usually involves treatment directed towards the causative etiology.[2] Topical corticosteroids are commonly used to treat corneal neovascularization.[2] With their broad anti-inflammatory properties, topical corticosteroids are generally most effective when started in the acute period of tissue injury and corneal neovascularization.[2] They have limited angio-regressive ability and are not effective for reversal of established or chronic corneal neovascularization.[2] Additionally, corticosteroids have numerous ocular side effects including delayed wound healing, increased susceptibility to certain infections, cataracts, and glaucoma. More recently, vascular endothelial growth factor inhibitors such as bevacizumab have been shown to be effective for the treatment of corneal neovascularization.[4] However, these agents are also more effective at treating acute rather than established or chronic corneal neovascularization.[4] Additional, non-pharmacologic treatments for corneal neovascularization including physical vascular ablation with laser, fine needle diathermy, and photodynamic therapy (PDT), have also been explored with variable results.[5]
Rationale for MICE
The rationale for MICE derives from principles of intravascular chemoembolization for the treatment of hepatocellular carcinoma (transarterial chemoembolization [TACE]). The first line treatment for intermediate stage hepatocellular carcinoma, TACE is based on the observation that hepatocellular carcinoma has a predominantly separate arterial blood supply compared to the rest of the liver which receives a venous supply.[6] TACE aims to induce more localized tumor necrosis through intra-arterial injection of a chemotherapeutic agent into the tumor circulation.[6] Mitomycin C (MMC) is a chemotherapeutic agent which has been used in TACE and is likely cytotoxic to vascular endothelial cells.[7] Thus, the rationale is that selective drug delivery of MMC to aberrant corneal vessels may locally deliver therapy to induce regression of corneal neovascularization and remove the nidus for lipid deposition.
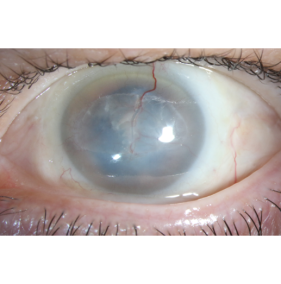
Indication and patient selection
The patient should have visually significant corneal neovascularization and lipid keratopathy approaching or in the visual axis (Figure 1). Depending on the underlying etiology of the corneal neovascularization, the patient should have undergone a full treatment course with documented failure of conventional treatment.
When preoperatively evaluating MICE candidacy, the vascular configuration should be evaluated to look for prominent and potentially larger caliber vessels (Figure 1). Such vessels may be more readily amenable to cannulation.
Surgical Techniques
The procedure should be done using the aid of an ophthalmic operating room microscope. Topical anesthesia followed by the usual pre-operative surgical prep should be applied to the operative eye.
A 1.0 cc TB syringe filled with mitomycin C (MMC) (0.4 mg/mL) and attached to a 33 or 34 gauge TSK needle (TSK Laboratory International, Canada) should be used.[1] It is not recommended to use a needle larger than 32 gauge.[1]
The surgeon should identify the largest bore corneal vessel just inside the limbus.[1] There is no requirement to identify if the vessel is efferent or afferent as there should be enough hydrostatic pressure from the mitomycin injection to fill and ablate both.[1]
The needle should be angled at approximately 15 degrees from the corneal surface to cannulate the corneal vessel.[1] The bevel of the needle should be completely intrastromal to prevent escape of MMC to the ocular surface.[1] The surgeon must also take care to avoid complete, full thickness penetration of the cornea and subsequent intra-ocular injection of MMC into the anterior chamber.
A small volume of MMC (0.01 to 0.05 mL) depending on size of the corneal vessel should be injected with enough hydrostatic force to allow for retrograde MMC propulsion through both afferent and efferent vessels.[1] Avoid injecting towards the limbus. The surgeon can look for blanching of the blood vessels during the procedure.[1] If the vessel does not blanch, it is likely the MMC has gone intrastromal and not intravascular.
Balanced salt solution (BSS) should then be used for meticulous irrigation of the ocular surface to remove any remnant MMC from the ocular surface.[1] Mitomycin C has known corneal epithelial toxicity, and although volumes of egressed fluid may be small, liberal irrigation helps to reduce potential risk for MMC surface toxicity.
Standard post-operative antibiotic and steroid drops or ointment should be applied to the operative eye at the conclusion of the case.
Outcomes and complications
Clinical outcome
Since MICE has only recently been described, published outcome data is limited,[1] but is anticipated to continue to emerge as use expands.
Ablation of the corneal neovascularization can be noted early on in the postoperative period including on post-operative day one.[1] In the weeks following treatment there can be residual lipid and blood in treated areas of the corneal stroma that will reabsorb. This absorption of lipid can result in flattening or compaction of the cornea with resultant astigmatism which should stabilize or improve in the postoperative period.[1] With successful embolization there has not been any reported cases of recurrence of corneal neovascularization although long-term follow up is limited.[1] If residual blood vessels remain, MICE can be repeated.[1]
Risks and complications
MICE is still new and long-term follow up and results are limited.[1] Injection should be directed away from the limbus to mitigate the risk of loss of normal limbal vasculature.[1] Following injection of MMC, the surgeon should copiously irrigate the ocular surface with BSS to reduce the risk of ocular surface exposure, toxicity, and melt. It is not known if MICE affects the corneal endothelial cells.
Conclusion
MICE is a novel treatment for visually significant corneal neovascularization and lipid keratopathy. As the procedure is relatively new, long-term results and adverse effects are not well known. However, as most current treatments are only effective at treating acute rather than establish or chronic corneal neovascularization and lipid keratopathy, MICE may be an effective and promising treatment option for appropriate patients.
References
- ↑ Jump up to: 1.00 1.01 1.02 1.03 1.04 1.05 1.06 1.07 1.08 1.09 1.10 1.11 1.12 1.13 1.14 1.15 1.16 Mimouni M, Ouano D. Initial outcomes of mitomycin intravascular chemoembolization (MICE) for corneal neovascularization. Int Ophthalmol 2022;42:2407–2416.
- ↑ Jump up to: 2.0 2.1 2.2 2.3 2.4 2.5 2.6 Nicholas MP, Mysore N. Corneal neovascularization. Experimental Eye Research 2021;202:108363.
- ↑ Jump up to: 3.0 3.1 Hall MN, Moshirfar M, Amin-Javaheri A, et al. Lipid Keratopathy: A Review of Pathophysiology, Differential Diagnosis, and Management. Ophthalmol Ther 2020;9:833–852.
- ↑ Jump up to: 4.0 4.1 Papathanassiou M, Theodoropoulou S, Analitis A, et al. Vascular Endothelial Growth Factor Inhibitors for Treatment of Corneal Neovascularization: A Meta-Analysis. Cornea 2013;32:435–444.
- ↑ Feizi S, Azari AA, Safapour S. Therapeutic approaches for corneal neovascularization. Eye and Vis 2017;4:28.
- ↑ Jump up to: 6.0 6.1 Raoul J-L, Forner A, Bolondi L, et al. Updated use of TACE for hepatocellular carcinoma treatment: How and when to use it based on clinical evidence. Cancer Treatment Reviews 2019;72:28–36.
- ↑ Hoorn CM, Wagner JG, Petry TW, Roth RA. Toxicity of Mitomycin C Toward Cultured Pulmonary Artery Endothelium. Toxicology and Applied Pharmacology 1995;130:87–94.

