Minimal Surgery for Retinal Detachment
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
Retinal detachment (RD) is defined as a separation between the neurosensory retina from the underlying retinal pigment epithelium (RPE). While RDs can be tractional or exudative in nature, they are most commonly rhegmatogenous (“rhegma” from Greek – break). RD is one of the important causes of sudden painless loss of vision and needs urgent repair in order to regain useful vision. Due to the enormous burden caused by this disease across the globe (1 in 10,000) and its economic stress on society, it is important to continue to explore ways of repairing RDs in as effective and non-invasive way as possible. Minimal surgery for retinal detachment, as made famous by Lincoff and Kreissig, is one option requiring only minimal extraocular intervention to treat RD.[1]
Disease Entity
Rhegmatogenous retinal detachment occurs essentially due to the presence of a break associated with vitreous liquefaction, traction and intraocular fluidics combining to overcome the physiological forces which keep the neurosensory retina apposed to the pigment epithelium.
Risk Factors
The most common ones are myopia, trauma, posterior capsular rupture during complicated cataract surgery, and retinal degenerations like lattice degeneration. Rarer ones include infectious retinitis, and hereditary vitreoretinal disorders. The pathophysiology, primary prevention and clinical features are discussed in detail on EyeWiki page Retinal detachment.
History of treatment options
The history of retinal detachment surgery can be broadly divided in to the pre (before 1920s) and post–Jules Gonin era (1930s and later). It was Gonin who first reported the successful treatment of detachment by sealing the retinal break to the underlying RPE and choroid.[1]. He was able to show that breaks were in fact the cause of the detachment and successful treatment would require their sealing. Gonin originally used a Paquelin thermal cautery to seal the break via vitreous.[2] The decades following his work usually involved variants of this method, with the success rate of reattachment being around 50%. Major advances included the use of intraocular air to seal breaks and the scleral resection experiments which later evolved into scleral buckling. The modern techniques can broadly be classified into retinopexy, scleral buckling, and vitreous surgery with intraocular tamponade. Here we highlight the scleral buckling evolution. Most of these are summarized from Dr. Kreissig’s work.[1]
Scleral Buckling
Mueller introduced shortening of sclera in 1903 to reduce the volume of the globe in the hope that the neurosensory retina and the RPE would appose due to a suction effect. This was modified and evolved into lamellar resection of sclera where two-thirds of the outer sclera were removed over the break in a circumferential manner. The sclera was inverted and closed with application of diathermy or later cryotherapy/photocoagulation. This in effect caused both shortening and a buckling effect in the manner of encirclage. 1937, Jess was the first one a foreign substance to create a scleral buckle. But the first buckling procedure using an exoplant was performed by Custodis in 1949. He applied surface diathermy to the full-thickness sclera over the break and sutured a polyviol material to the sclera. The eye wall was indented at the area of the break so that the retina would appose the RPE and close the break. In 1956, he reported a case series of about 500 patients with a success rate of 83.3% reattachment.[3] He did not believe in the need to drain the fluid in order to have successful reattachment and this was the beginning of the minimal surgery (extraocular only). Schepens used and popularized the silicone exoplants but was inclined to drain the subretinal fluid, contrary to Custodis. It was in 1965 that Brockhorst and colleagues described the classical buckling procedure involving lamellar dissection and cerclage using various silicone explants.[4] The next major work was done by Lincoff, from the replacement of Custodis’ poluvinyl exoplants with silicone, introduction of better needles, and the movement from diathermy to cryopexy.[5] The non-drainage, minimal surgery for retinal detachment was further refined and popularized by Kressig, through the turn of the century to present day.[6]
Definitions
The ideal procedure for treatment of rhegmatogenous detachments should be one involving closure of breaks with minimum of trauma, maximum of primary attachment, minimum of reoperations and complications, and maximum long term sustenance along with good functional results. The basic principles for all types of surgeries for rhegmatogenous detachments are:
- Identification of all retinal breaks and areas of vitreoretinal traction (if present)
- Induction of aseptic chorioretinal inflammation around the breaks to seal them
- Ensuring chorioretinal apposition for at least a few weeks by either:
- External tamponade (scleral buckle, balloon buckle)
- Internal tamponade (air, gases, or liquids like silicone oil)
These are constant across the various options available for rhegmatogenous detachment repair:
- “Classical scleral buckling” involving cryopexy, encerclage or 360 ° circumferential buckle along with drainage of subretinal fluid. (creation of barriers and volume reduction)
- “Pneumatic retinopexy” a minimally invasive technique using intravitreal gas injection along with cryopexy or laser along with post-operative positioning
- “Primary vitrectomy” (with or without scleral buckling), along with endolaser and silicone oil tamponade (using high-speed cutters – 2500/min, panoramic viewing systems and perfluoro-carbon liquids)
- Minimal surgery (Minimal Segmental Buckling with Sponges and Balloons Without Drainage - “Extraocular Minimal Surgery”) using limited scleral buckle with cryopexy without drainage
The choice of surgery depends on multiple factors, from patient pathological nature of detachment, associated traction, affordability to equipment availability and surgeon comfort. We discuss here the Minimal Segmental Buckling with Sponges and Balloons without Drainage which we will refer to as "minimal extraocular surgery" from this point forward. This technique boasts proven and sustained long term results, but remains an underutilized option.
Evidence
Despite being promulgated decades ago, minimal extraocular surgery for retinal detachment remains a rather hidden gem. This section is dedicated to highlight the scientific data and enhance awareness of this procedure as a treatment option.
A Medline analysis presented by Dr. Kreissig using terms “retinal detachment,” “segmental buckling,” “minimal extraocular surgery,” and “nondrainage” were analyzed in English, German, Italian, French, Spanish, and in some East European journals. The analysis consists of five reported series, despite having certain differences with case selection and other parameters, with a combined total of 1,462 retinal detachments. In summary, it was found that minimal segmental buckling limited to the area of break with non-drainage approach, resulted anatomically in primary retinal reattachment of about 91% cases and after reoperation, 97.4% of cases which persisted in a 2-year follow-up.[6][7] [8][9][10][11] Functional results were more difficult to compare, especially as follow-up was varied, but essentially confirmed that minimal segmental buckling has no negative effect on long-term visual function.
The great challenge for comparing minimal extraocular surgery with other techniques is the variability in patient selection, surgeon factors, types of steps in each category of surgical choice undertaken, and the long-term follow-up. It should be acknowledged that the initial cases were probably not similar as there are varied selection criteria each of these studies.
| Procedure | Rate of Retinal
reattachement after single surgery (%) |
Long term follow up
with redetachment (%) |
Long term follow up
with redetachment (%) |
No of years | References |
|---|---|---|---|---|---|
| Minimal (extraocular
– non drainage) surgery for retinal detachment |
91 | 97.4 | 12.1 | 15 | [6][7][8][9][10][11][12] |
| Primary vitrectomy
(with or without Buckle) |
87.7 | 96.7 | NA | NA | [13][14][15][16][17] |
| Pneumatic retinopexy | 75.5 | 97.4 | 17.2 | NA | [18][19][20][21] |
| Scleral buckling with
encirclage and drainage (classical buckling) |
78 | 96 | NA | NA | [22][23] |
Again, it must be clearly reiterated that the table is not a true comparison of the various techniques as there are far too many variables in the individual studies involved which would undoubtedly create bias. However, individually it seems to demonstrate that most of the procedures have good anatomical success rates reaching above ninety percent for most options. Further, the question of efficacy and safety with regards to second surgery and PVR are important considerations. As per the systematic review by Dr. Kreissig, the pooled risk of second surgery for primary vitrectomy was 13.3%, for pneumatic retinopexy 25.2%, and for segmental buckling 9.1% while that of PVR after vitrectomy was 5.3%, after pneumatic retinopexy was 6.5%, and after scleral buckling 0.9%.[1]
The question of cost
Another important consideration lies in the fact that retinal detachment occurs irrespective of the socioeconomic condition of the individual across the globe. Considering that nearly half of the world population lives on less than $5.50 a day according to the World Bank [24], it is even more of a problem in the developing countries. It is important to consider the cost associated with management options, especially when treatment of a disease like RD involves long-term care and possibly multiple surgeries. Vitrectomy, though is becoming popular, can involve significantly larger costs compared to buckling or retinopexy, especially with the management of lens, glaucoma and silicone oil issues.
Case selection & limitations
Minimal segmental buckling with non-drainage can be successfully used to treat nearly 90 % of rhegmatogenous detachments. Detachments with multiple breaks (if same latitude) and even with proliferative vitreoretinopathy (PVR) grade C1–C2 can be treated with segmental buckling and without drainage (Figs. 6.4–6.8). The segmental buckles may consist of radials, short circumferentials, or a combination of both, but without a cerclage. Limitations do exist. In the nearly ten percent remaining types of detachment, minimal segmental buckling is difficult and should probably be avoided in situations with:
- Multiple tears at different latitude (2-3%)
- Posterior tears (about 1%)
- Circumferential retinal detachment with extent greater than 70 °
- Giant retinal tears
- Star folds near (within 1 clock hour) the area to be buckled
- Local vitreous traction, PVR greater than C2
- Poor visualization
Pre-surgical workup
Finding the breaks
As the basic principles of detachment surgery enshrines, the most important aspect of any and all types of reattachment surgery involves localization of all the leaking breaks accurately. Being true to the cause of detachment, as elucidated by Gonin and Custodis, Minimal surgery depends on this essential step, perhaps even more so than the actual steps of surgery. Meticulous examination with indirect ophthalmoscope, using scleral depression as well as biomicroscopic slit lamp examination with Goldmann lens for phakics/ aphakic and the widefield contact lens for psuedophakics with scleral depression are is critical.[25][26] Concentrate on finding the primary break--the one alone causing the configuration of the detachment. Usually the configuration of the detachment mainly depends on the primary break and gravity exerted on the subretinal fluid.
The 4 rules originally created by Lincoff by analysing nearly a thousand detachments still hold true:
Rule 1: Superior temporal or nasal detachments: The primary break that determines the shape of the detachment lies within 11/2 clock hours beneath the higher border of the detachment in 98%
Rule 2: Superior detachments that cross the midline at 12 o’clock and advance down both sides of the disk eventually become a total detachment: In this type of detachment, the primary break will lie in a triangle the apex of which is at 12 o’clock and its sides are 11/2 clock hours to either side of 12 o’clock in 93%
Rule 3: Inferior detachments: The higher border of the detachment indicates the side of the disk on which the inferior break is to be found in 95%
Rule 4: Inferior bullae in rhegmatogenous detachments: Originate from a superior break
Later Dr. Kreissig expanded on Dr. Lincoff's work for finding the break in a redetachment after a failed buckle.[26]
Reoperation Rule 1: When the superior border of a temporal or nasal superior detachment drops below the buckle, it implies an undetected break within 11/2 clock hours below the new superior border.
Reoperation Rule 2: When the pattern of a detachment (superior, lateral or inferior) converts from one pattern to another, it indicates an undetected break consistent with the new pattern.
Reoperation Rule 3: When the borders of a detachment remain unchanged after a buckling operation and the buckle is in the correct position, this implies an undetected break above the buckle.
Reoperation Rule 4: When a total detachment remains unchanged after being encircled and drained, it implies an undetected break anterior to the existing cerclage near 12 o’clock.
It must be recalled that additional breaks that might be present in addition to the primary break.
- 1 break in about 50% of detachments, but
- 2 breaks in 30% and
- 3 breaks in 20%,
- and that they tend to be located within 1 quadrant of the primary break.[27]
Orientation of the segmental buckle plan:
It must be remembered that basic anatomy of vitreous attachments to the normal retina and the spherical globe makes the tractional forces more acute in a radial manner. By simple physical laws, a circumferential buckle would also lead to augmentation of the radial folds as it shortens the retina circumferentially. These radial folds can aggravate the break resulting in fishmouthing. Hence the approach should be to orient a radial buckle, preferably a sponge such that it covers the posterior edge of the break as well as supports the operculum appropriately, which can lead to later traction.[28] Several radial buckles can be used in combination for multiple breaks, but the breaks have to be separated by at least 1 ½ clock hours. If the breaks are closer, then circumferential buckle can be used. Goldbaum calculated that when applying a circumferential buckle less than 90 °, the radial folds are less likely, hence can be used for multiple breaks in the same latitude.[29] For more complex detachments and redetachments, balloon tamponade as well as scleral pouch has been described in detail by Dr. Kreissig.[27][30]
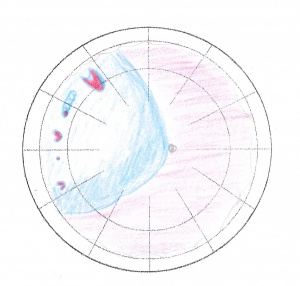
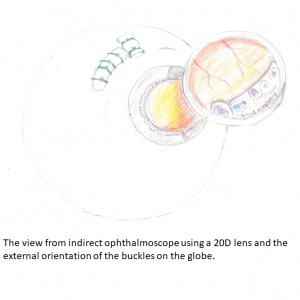
Other prerequisites
Finding and documenting the various vitreoretinal adhesions and grading proliferative vitreoretinal changes (PVR) is important. Irrespective of the classification used, it should be consistent and well elucidated. The availability of equipment, both before and during surgery is a must; this equipment includes standard retinal instruments, cryo probes for retinal surgery, non-absorbable spatulated 5-0 sutures along with silicone sponges and bands of various sizes, depending on the surgeons’ preference. Patient counseling about the difference between anatomical and functional results, along with the possibility of future surgeries and precautions for the remaining eye are a must, beyond the usual discussion regarding guarded visual prognosis.
Surgery
Principles
After finding the break, the next major principle is to close all the breaks. This occurs due to localized indentation of the sclera, choroid and RPE beneath a retinal break which leads to alteration of anatomical and physiological factors. It is usually achieved with cryopexy and is maintained by accurate tamponade using segmental buckle. This leads to closure of the break which causes the normal physiological forces can maintain a permanent state of attachment.
In a non-drainage procedure, functional closure occurs due to many possible mechanism of scleral buckling:
- reduction of vitreoretinal traction by displacing the eye wall and retina centrally;
- displacement of subretinal fluid away from the location of the retinal break and scleral buckle;
- postoperative increase in the height of the scleral buckle;
- approximation of the retinal break and adjacent vitreous gel;
- increase in resistance to fluid flow through the retinal break, with consequent increase in the relative reattachment forces; and
- alteration in the concave shape of the eyeball, resulting in a change in the effect of intraocular currents that encourage liquid vitreous to enter the subretinal space.
These effects are probably additive and work together even in drainage cases. Although contemporary scleral buckling procedures routinely include the creation of a chorioretinal burn with cryotherapy or laser to induce adhesion from reactive scaring, such an adhesion is not always necessary to maintain retinal reattachment.[31]
Steps of surgery
Local anaesthesia is usually preferred with a peribulbar or retrobulbar injection. General anaesthesia is used in children and uncooperative patients.
Limbal peritomy, at least 1 ½ clock hours on either side of the area of break, or 360° with meridional cuts. Traction sutures are placed under all four rectus muscles.
On table, examination should be done and compared to pre-operative notes. Marking of the breaks is done, both of the antero-posterior and lateral edges (three-point localization). The position of the buckle depends on the precise marking of the break edges on the sclera, which usually requires a good assistant.
Cryosurgery unit is set at –40° to –60° C at the tip and checked before starting Every cryotherapy application should be monitored ophthalmoscopically, should be done minimally, and stopped as soon as the desired freeze happens. Diagnostic cryo should be only attempted if other methods do not work for finding the break and should be kept to a minimum.
The mattress suture (5-0 Dacron or Rd-1 Mersilene with 2 spatula needles) should be placed symmetrically on either side of the anterior and posterior localizing marks, and should overlap the 2 lateral marks by 1-2 mm. The distance between the 2 limbs of the mattress suture defines the diameter of the sponge to be selected. The distance between the sutures as well as the tightness determines the indentation obtained from the buckle, but the former is preferred to adjust the amount of the same. The mattress sutures should have long intrascleral limbs rather than short multiple passages as the latter ones tend to tear out. The second important consideration is the placement of the sutures. If one is uncertain, it is helpful to indent the anterior portion of the tied buckle with the closed tips of the blunt forceps. If the buckle is supporting the anterior portion of the break sufficiently, it can be concluded (taking into consideration the posterior position of the suture that has been placed) that the posterior part of the break will be tamponaded as well.
| Retinal break | Radial Sponge | Width of mattress
sutures |
|---|---|---|
| 2mm | 4mm | 6mm |
| 3mm | 5mm | 8mm |
| 5mm | 7.5 (x 5) mm | 10mm |
| 8mm | Two 7.5 (x 5) mm | 14mm |
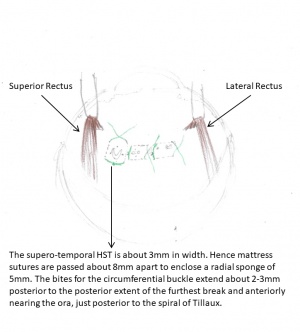
After tying the sutures, check the central retinal artery for patency. If the central artery is closed, digital massage of the eye will accelerate reopening of the artery. Most arteries will begin to show pulsation in 2-3 minutes. If there is no sign of pulsations within 5 minutes, a tied suture can be reopened or part of the compressed sponge can be pushed back under the tied suture. However, if there is still no sign of pulsation after another 5 minutes, the eye is probably one with poor outflow, and paracentesis or drainage of subretinal fluid is indicated.
When multiple sutures needed, check central retinal artery after each knot.
Tips for minimal surgery, especially important for secure conjunctival closure over explant:
- Avoid grasping the conjunctiva with sharp instruments.
- When eyeball rotation is needed, use traction sutures.
- Fluid or blood swabbed gently at a distance, avoid touching other tissues.
Confirm with ophthalmoscopy that the buckle is in proper position and central retinal artery looks perfused.
Close Tenon’s and conjunctiva. Prior closure to surgical field, rinse with antibiotic solution, and apply atropine 1% with an antibiotic/steroid ointment. Patch the eye for about 24 hours.
Topical antibiotics and steroids should be tapered in the postoperative period as per usual protocol. No systemic antibiotics are needed. Special exercises for the prevention of diplopia is advised by some physicians such that starting on the first postoperative day, the patient has to practice binocular motility exercises in all directions daily 5-10 times for about 3 minutes. This is continued for about a month.[30]
Complications
Complications of scleral buckling & cryotherapy
Discussed in the EyeWiki article on scleral buckling in detail.
Complications specific to minimal extraocular surgery using non-drainage approach
Intraoperative
Central retinal artery non-perfusion: Caused by sudden rise in IOP after tightening of sutures. Management outlined above.
Post-operative
Sponge extrusion/ infection – less than 1 %
Diplopia – less than 1 % with postoperative motility exercises
Failure
Reasons of primary and final failure can be listed as:
- Missed breaks
- Inadequate buckles
- Proliferative vitreoretinopathy
Conclusion
The four approaches for treatment of retinal detachment all depend on accurate localization of breaks. Pneumatic retinopexy, drainage with buckling and vitrectomy are all intraocular while minimal approach using non-drainage is extraocular. Most of these have an anatomical success rate of 94-99% with varying degrees of morbidity.
The approach towards minimal extraocular intervention using non-drainage technique offers an effective and relatively affordable tool in the management of retinal detachment. Moreover, the remarkable settlement of the retina and the absorption of subretinal fluid without drainage within 24 hours is itself an indicator of the success of surgery. Since the surgery is minimal, so are the complications.
The success of minimal extraocular surgery requires diligent work-up and meticulous precision along with great patience on the part of the surgeon and patient, but the outcomes can be overwhelmingly positive. As Dr. Kreissig concludes in one of his publications, "A minimum of extraocular surgery for a rhegmatogenous detachment can only provide a maximum of success if it is preceded by a maximum of search for the break or breaks.” [1]
Additional Resources
- American Academy of Ophthalmology: The repair of rhegmatogenous retinal detachment. Information Statement. Ophthalmology 1990;97:1562–1572.
- Brinton DA, Wilkinson CP. Retinal Detachment. Principles and practice. 3rd Edition. 2009; New York, Oxford University Press, pp149-180.
- Kreissig I (2000) A practical guide to minimal surgery for retinal detachment: volume 1. Diagnostics – segmental buckling without drainage – case presentations. Thieme Stuttgart, New York, pp 1–300
- Kreissig I (2000) A practical guide to minimal surgery for retinal detachment: volume 2. Temporary tamponades with balloon and gases without drainage – reoperation – case presentations. Thieme Stuttgart, New York, pp 1–368
- Ingrid Kreissig (Ed.) Primary Retinal Detachment: Options for Repair, 1e, Springer, 2005
References
- ↑ 1.0 1.1 1.2 1.3 1.4 Ingrid Kreissig (Ed.) Primary Retinal Detachment: Options for Repair, 1e, Springer, 2005
- ↑ Gonin (1920) Bull Soc Franc Ophthal 33:1
- ↑ Custodis E (1956) Die Behandlung der Netzhautabloesung durch umschriebene Diathermiekoagulation und einer mittels Plombenaufnaehung erzeugten Eindellung der Sklera im Bereich des Risses. Klin Monatsbl Augenheilkd 129:476
- ↑ Brockhurst RJ (1965) Scleral buckling techniques. In: Schepens CL, Regan CDJ (eds) Controversial aspects of the management of retinal detachment. Little, Brown, Boston, p 111
- ↑ Lincoff HA, Baras I, McLean J (1965) Modifications to the Custodis procedure for retinal detachment. Arch Ophthalmol 73:160
- ↑ 6.0 6.1 6.2 Kreissig I, Rose, D, Jost B (1992) Minimized surgery for retinal detachments with segmental buckling and nondrainage: an 11-year followup. Retina 12: 224–233
- ↑ 7.0 7.1 Kreissig I, Failer J, Lincoff H, Ferrari F (1989) Results of a temporary balloon buckle in the treatment of 500 retinal detachments and a comparison with pneumatic retinopexy. Am J Ophthalmol 107:381–389
- ↑ 8.0 8.1 Lincoff H, Kreissig I, Goldbaum M (1974) Reasons for failure in nondrainage operations. Mod Probl Ophthal 12:40–48
- ↑ 9.0 9.1 Kreissig I, Simader E, Fahle M, Lincoff H (1995) Visual acuity after segmental buckling and non-drainage: a 15-year follow-up. Eur J Ophthalmol 5:240–246
- ↑ 10.0 10.1 Sivkova N, Katsarov K, Kreissig I, Chilova-Atanassova B (1997) Our experience in minimized surgery for retinal detachment: first results. Folia Medica 34: 44–47
- ↑ 11.0 11.1 Sirtautiene R, Bagdoniene R (1997) Minimised surgery for retinal detachments with segmental buckling and non drainage. In: XIth Congress of the European Society of Ophthalmology, Budapest 1997. Monduzzi Editore S.p.A., Bologna, pp 1161–116
- ↑ Kreissig I, Simader E, Fahle M, Lincoff H. Visual acuity after segmental buckling and non-drainage: a 15-year follow-up. Eur J Ophthalmol 1995; 5: 240-6.
- ↑ Heimann H, Bornfeld N, Friedrichs W, Helbig H, Kellner U, Korra A, Foerster MH (1996) Primary vitrectomy without scleral buckling for rhegmatogenous retinal detachment. Graefes Arch Clin Exp Ophthalmol 234:561–568
- ↑ Bartz-Schmidt KU, Kirchoff B, Heimann K (1996) Primary vitrectomy for pseudophakic retinal detachment. Br J Ophthalmol 80:346–349
- ↑ Campo RV, Sipperley JO, Sneed SR, Park DW, Dugel PV, Jacobsen J, Flindall RJ (1999) Pars plana vitrectomy without scleral buckle for pseudophakic retinal detachments. Ophthalmology 106:1811–1815
- ↑ Devenyi RG, de Carvalho Nakamura H (1999) Combined scleral buckle and pars plana vitrectomy as a primary procedure for pseudophakic retinal detachments. Ophthalmic Surg Lasers 30:615–618
- ↑ Newman DK, Burton RL (1999) Primary vitrectomy for pseudophakic and aphakic retinal detachments. Eye 13:635–639
- ↑ Algvere P, Hallnaes K, Palmqvist BM (1988) Success and complications of pneumatic retinopexy. Am J Ophthalmol 106:400–404
- ↑ Sebag J, Tang M (1993) Pneumatic retinopexy using only air. Retina 13:8–12
- ↑ Eter M. Boeker T, Spitznas M (2000) Long-term results of pneumatic retinopexy. Graefes Arch Clin Exp Ophthalmol 238:677–681
- ↑ Holz ER, Mieler WF (2003) Controversies in ophthalmology: view 3: the case for pneumatic retinopexy. Br J Ophthalmol 87:787–789
- ↑ McAllister IL, Meyers SM, Zegarra H, Gutman FA, Zakov ZN, Beck GJ (1988) Comparison of pneumatic retinopexy with alternative surgical techniques. Ophthalmology 95:877–883
- ↑ Han DP, Mohsin NC, Guse CE, Hartz A, Tarkanian CN, Southeastern Wisconsin Pneumatic Retinopexy Study Group (1998) Comparison of pneumatic retinopexy and scleral buckling in the management of primary rhegmatogenous retinal detachment. Am J Ophthalmol 126:658–668
- ↑ https://www.worldbank.org/en/news/press-release/2018/10/17/nearly-half-the-world-lives-on-less-than-550-a-day
- ↑ Lincoff H, Gieser R. Finding the retinal hole. Mod Probl Ophthalmol 1972; 10: 78-87.
- ↑ 26.0 26.1 Lincoff H, Kreissig I. Finding the retinal hole in the pseudophakic eye with detachment. Am J Ophthalmol 1994; 117: 442-6.
- ↑ 27.0 27.1 27.2 Kreissig I (2000) A practical guide to minimal surgery for retinal detachment: volume 1. Diagnostics – segmental buckling without drainage – case presentations. Thieme Stuttgart, New York, pp 1–300
- ↑ Lincoff H, Kreissig I (1975) Advantages of radial buckling. Am J Ophthalmol 79:955–957
- ↑ Goldbaum MH, Smithline M, Poole TA, Lincoff HA (1975) Geometric analysis of radial buckling. Am J Ophthalmol 79:958–965
- ↑ 30.0 30.1 Kreissig I (2000) A practical guide to minimal surgery for retinal detachment: volume 2. Temporary tamponades with balloon and gases without drainage – reoperation – case presentations. Thieme Stuttgart, New York, pp 1–368
- ↑ https://eyewiki.aao.org/Scleral_buckling_for_rhegmatogenous_retinal_detachment
- ↑ Wilkinson CP, Rice TA (1997) Michels retinal detachment, 2nd edn. Mosby St. Louis MO