Intraocular Lens (IOL) Tilt and Astigmatism
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
Introduction
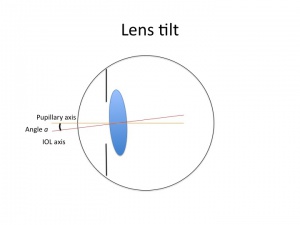
In modern cataract surgery with implantation of intraocular lenses (IOLs), IOL tilt has the potential to induce astigmatism and higher-order aberrations. Lens tilt is defined as the angle between the IOL optical axis and the baseline axis. Decentration is defined as the vertical distance from the IOL center to baseline [1]. The simple aberration theory suggests that a beam of light entering a spherical lens obliquely will produce marginal oblique astigmatism. IOL tilt is usually at a magnitude of about 5-6 degrees and usually greatest around a vertical axis with the nasal edge displaced anteriorly and temporal edge displaced posteriorly [1]. IOL tilt is becoming increasingly important with the rising popularity of scleral-fixated IOLs such as those implanted using the Yamane technique or other methods of scleral fixation.
Theoretical Astigmatism Induced by Lens Tilt
A thin lens of power P in air, in the pupillary plane, and tilted by angle a has astigmatism A with an angle perpendicular to the tilt axis. This is summarized in the following equation [1]:
A=[P[1+(sin a)2/(3)]] x (tan a)2
A is astigmatism in diopters and P is IOL power in diopters.
Theoretical simple computers have limitations to these calculations, but customized pseudophakic computer eye models can overcome these limitations. These eye models include anterior surface topography, anterior chamber depth, axial length, IOL tilt and decentration, foveal misalignment and IOL geometry.
Computer models that use ray tracing compared estimated/simulated astigmatism to the actual measurements of aberrations on the same eyes and found a consistent overmeasurement of simulated astigmatism. The cornea may be the main source of astigmatism in pseudophakic eyes with clinically normally placed IOLs, which suggests that perhaps IOL tilt plays a negligible role [1]. This likely differs significantly in scenarios where lens centration is suspect or atypical.
Incidence
Lens tilt or decentration occurs in about 5-10% of patients after scleral-sutured posterior chamber lens implantation [2][3] and can occur even after uncomplicated implantation.
Signs and symptoms
Large amounts of IOL tilt or decentration may cause enough astigmatism to significantly affect quality of vision. Guyton et al. found that 1 mm of decentration and greater than 5 degrees of tilt optically alter visual acuity [4]. Multifocal, toric and toric multi-focal are more sensitive to small changes in tilt compared to monofocal IOLs and centration parameters need to be particularly accurate and precise.
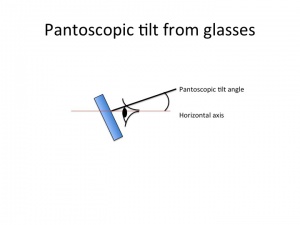
Lens tilt with spectacles
Tilting a lens along the horizontal axis (180 axis) is called pantoscopic tilt. Tilting along the vertical axis is called face form tilt.
Tilting a lens with the top forward and the bottom backward will increase plus sphere power for a plus lens or will increase minus power for a minus lens. Thus, the prescription of the glasses will change when tilted.
For a plus lens, tilting at 180 axis will increase the positive sphere power and will add positive cylinder power in the 180 axis.
For a minus lens, tilting at 180 axis will add negative sphere power (the sphere will become more minus), increase minus cylinder at 180 axis but will increase plus cylinder in the 90 axis. Therefore, tilting glasses at 180 axis can ameliorate under-corrected myopia or under corrected with-the-rule astigmatism.
Causes of IOL tilt
Uneventful cataract surgery, zonular abnormalities, suture fixation, or haptic-optic asymmetric positioning can cause IOL tilt. In scleral-sutured IOLs, multiple factors such as IOL haptic positioning, suturing errors, lack of capsular support, scleral tunnel positioning, and haptic breakage can contribute to IOL tilt. Among scleral fixated IOLs, one recent study compared sutured vs sutureless scleral fixated lenses and found that sutureless IOLs had less tilt and induced astigmatism. [5]
Evaluation
IOL tilt can be measured with ultrasound, the Purkinje reflection device, the Scheimpflug camera, anterior segment OCT, or subjective grading at the slit lamp with findings including transillumination defects.
Measurement of IOL Tilt
Purkinje imaging
Purkinje imaging uses light reflections from the ocular surface of the eye to evaluate alignment. Light is reflected at each media interface with a difference in refractive index [6]. The images are reflections from the anterior corneal surface (PI), posterior corneal surface (PII), anterior lens surface (PIII), and posterior lens surface (PIV). This method assumes that the locations of images of a light source are linearly related to eye rotation (B), IOL tilt (a) and IOL decentration (d), and relies on proper measurements of lens radius of curvature [1].
PI=A(B)
PIII=B(B)+C(a)+(D)d
PIV = EB +Fa +Gd
A-G are coefficients customized to the anatomic parameters of the eye [1].
Scheimpflug imaging
Scheimpflug imaging provides anterior segment images with a large depth of focus. This scanning method is quick, requiring patients to fixate for 1.5 seconds without moving. The images are processed to obtain the pupillary axis, IOL axis, and IOL tilt. However, good pupil dilation (more than 6 mm) is required, which can affect the pupil axis and tilt characterizations. Identifying the structures needed to serve as a reference point can be difficult. Scheimpflug imaging is also subject to optical distortion (due to viewing anterior refracting surfaces) and geometric distortion (due to tilt of planes) [7]. This method is not used as much due to corneal magnification leading to inaccurate results [8].
Anterior segment optical coherence tomography (OCT)
Anterior segment OCT is a fast, noninvasive, non-contact and high-resolution method of measuring IOL tilt. The eye is scanned at the rotated axis which passes through the anterior corneal center and the pupillary center [1]. Advantages are the ability to be performed in the immediate postoperative period and in eyes with corneal opacification, such as from corneal edema, and it also does not need coupling fluid. The disadvantage is inability to visualize the haptic below the iris [9] [10].
Ultrasound biomicroscopy
Ultrasound biomicroscopy allows visualization of the tilt and decentration but may make quantification of IOL tilt challenging. This is a contact method and may deform the eye though this may be avoidable with certain precautions [1]. This typically requires elevated level of technical skill for both surgeons and ancillary staff.
Management
Prevention of induction of IOL tilt is possible intra-operatively. Use of capsular tension rings in case of poor zonular support can help prevent IOL tilt. For scleral fixated lenses, four point fixation is generally considered better to prevent tilt. In two point fixation, it is important to ensure that the sclerotomies are exactly 180 degree apart.
Indications for exchange, repositioning, or removal of the IOL include Uveitis-Glaucoma-Hyphema (UGH) syndrome, high astigmatism, and substantial increase in both lower and higher order optical aberrations.
The increasing use of intraoperative OCT for in vivo anatomic assessment and other imaging devices such as intraoperative aberrometry to detect refractive effects of pantoscopic tilt may allow surgeons to identify tilt in the operating room and adjust accordingly at the time of the initial surgery. This may assist in decreasing the incidence of tilt after cataract surgery, improving visual outcomes and potentially avoiding second surgeries.
References
- ↑ Jump up to: 1.0 1.1 1.2 1.3 1.4 1.5 1.6 1.7 Hoffer KJ. Marcos, Susana. IOL Power. Chapter 40: Special Circumstances: Effect of IOL Tilt on Astigmatism. (p. 223-230) 2011 Slack Incorporated.
- ↑ Sundmacher R., Althaus C., Webster R., et al: Two years experience with transscleral fixation of posterior chamber lenses. Dev Ophthalmol 1991; 22: pp. 89-93
- ↑ Hayashi K., Hayashi H., Nakao F., et al: Intraocular lens tilt and decentration, anterior chamber depth, and refractive error after trans-scleral suture fixation surgery. Ophthalmology 1999; 106: pp. 878-882
- ↑ Guyton, D. L., Uozato, H. and Wisnicki, H. J. (1990). Rapid determination of intraocular lens tilt and decentration through the undilated pupil. Ophthalmology 97, 1259-1264
- ↑ S Sul etal. Comparison of decentration, tilt and lenticular astigmatism of ıntraocular lens between sutured and sutureless scleral fixation techniques. J Fr Ophtalmol. 2021 Oct;44(8):1174-1179
- ↑ Nishi, Yutaro, et al. “Reproducibility of Intraocular Lens Decentration and Tilt Measurement Using a Clinical Purkinje Meter.” Journal of Cataract and Refractive Surgery., vol. 36, no. 9, pp. 1529–1535.
- ↑ De Castro, Alberto, et al. “Tilt and Decentration of Intraocular Lenses in Vivo from Purkinje and Scheimpflug Imaging. Validation Study.” Journal of Cataract and Refractive Surgery., vol. 33, no. 3, pp. 418–429.
- ↑ J. Tabernero, P. Piers, A. benito, M. Redondo, P. Artal. Predicting the optical performance of eyes implanted with IOLs to correct spherical aberration. Invest Ophthalmol Vis Sci, 47 (2006), pp. 4651-4658.
- ↑ Kumar, Dhivya Ashok, et al. “Evaluation of Intraocular Lens Tilt with Anterior Segment Optical Coherence Tomography.” American Journal of Ophthalmology., vol. 151, no. 3, pp. 406–412.e2.
- ↑ Wang L, Guimaraes de Souza R, Golla A, Weikert MP, Koch DD, “Evaluation of Crystalline Lens and IOL Tilt using Swept-Source Biometry,” presented at the ASCRS Symposium on Cataract, IOL and Refractive Surgery, Los Angeles, California, USA, May 2017