Infectious Crystalline Keratopathy
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
Disease Entity
Infectious Crystalline Keratopathy (ICK) is a rare infection of the cornea that results in grey-white, branching stromal opacities in the cornea with little surrounding inflammation. It has been associated with long-term topical steroid therapy and is most commonly found in patients who have had previous penetrating keratoplasty[1].
Etiology
Multiple organisms, including both fungal and bacterial entities, have been implicated in ICK.
However, the most common inciting organism is
- alpha hemolytic Streptococcus viridians[1].
Other organisms reported include:
- Streptococcus Pneumoniae,
- coagulase-negative Staphylococcus,
- Peptostreptococcus,
- Haemophilus species,
- Mycobacterium species,
- Pseudomonas,
- Stenotrophomonas,
- Citrobacter,
- Acinetobacter,
- Alternaria,
- Acanthamoeba,
- Enterobacter,
- Enterococcus species,
- Candida species,
- Serratia marcescens,
- Gemella haemolysans, and
- Actinomyces species[2].
Risk Factors
The presence of an immunocompromised corneal state, as is present with long-term topical steroid therapy, is a known risk factor for the development of infectious crystalline keratopathy. Though rare, the most common clinical setting for ICK is following penetrating keratoplasty. Other risk factors include corticosteroid use, contact lens wear, and other previous corneal surgeries [3]. In addition to penetrating keratoplasty, ICK has also been reported following lamellar keratoplasty, corneal relaxation incisions, Lasik, cataract extraction, and topical anesthetic abuse[2].
Pathophysiology
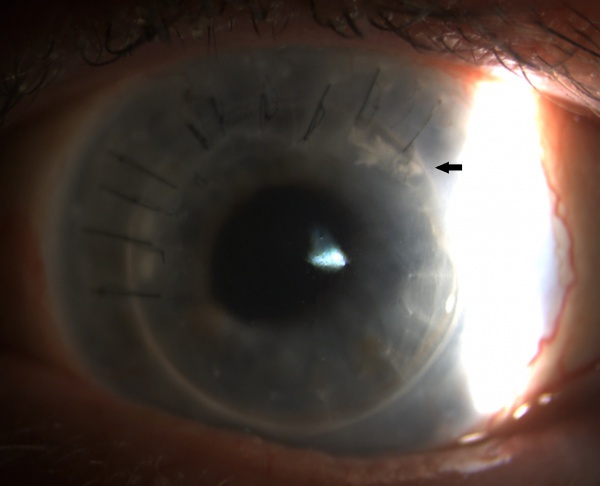
One of the important characteristics of ICK that has been described in the literature is the presence of biofilm. Compared to ICK, biofilms are rarely observed in other forms of chronic infectious bacterial keratitis. The presence of a biofilm in ICK has been demonstrated using Transmission Electron Microscopy (TEM) with specialized fixatives. The biofilm stains intensely with periodic acid-Schiff stain due to the polysaccharide-rich extracellular matrix and stains weakly with Gram stain. The biofilm seen in ICK is believed to be secreted as the organism’s response to the nutrient deprived environment in which it is found[4]. The biofilm is hypothesized to be responsible for the scant inflammatory response classically observed in ICK and the difficulties seen related to eradication and treatment. Additionally, the bacteria is thought to gain access into the stroma through an epithelial defect or epithelial ingrowth, often extending from a suture track (Fig 1)[2].
History
The first case of ICK was described by Gorovoy et al. in 1983 where needle like stromal opacities were observed within a corneal graft following penetrating keratoplasty. Analysis of the corneal button demonstrated epithelial ingrowth along a suture tract, and local colonies of gram-positive cocci diffusely throughout the corneal stroma. No inflammatory reaction was found within these areas despite the bacterial colonization[4].Thereafter, Meisler et al. described the first case series of three patients where a similar keratopathy developed while patients were using topical corticosteroids [5]. Since its discovery, many other reports have described cases of ICK in association with various other organisms. These reports have helped to establish risk factors associated with the development of ICK and treatment options for managing this rare infection.
Signs & Symptoms
Clinical signs and symptoms of infectious crystalline keratopathy usually emerge months to years following penetrating keratoplasty and follow an indolent clinical course. Symptoms can range from mild discomfort to severe pain, decreased vision, redness, photophobia, and swelling [6].
Physical Examination
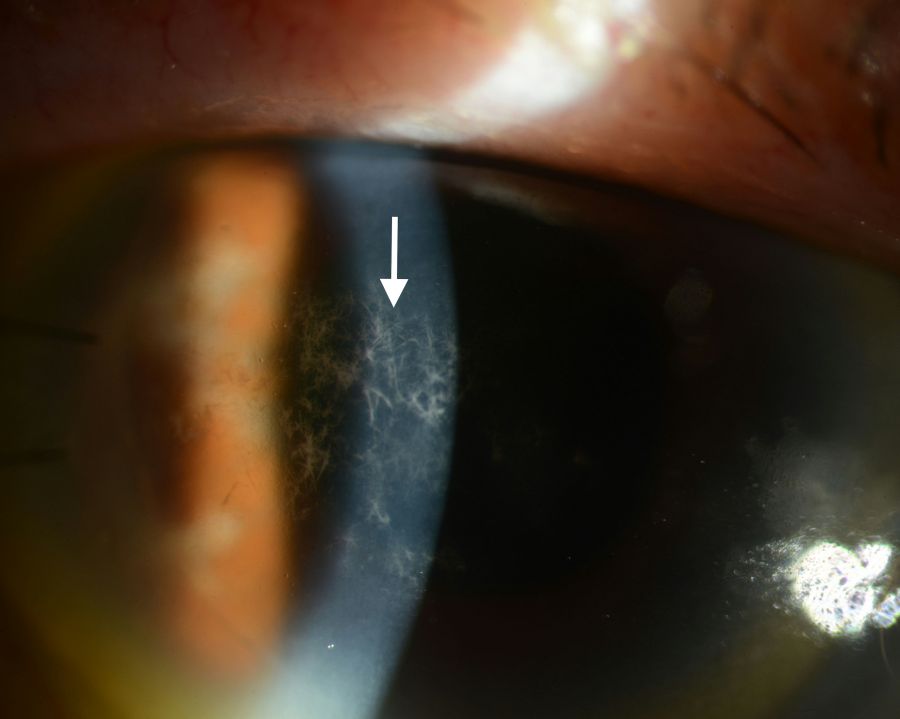
Physical examination may reveal chemosis and conjunctival injection. Additionally the presence of an anterior stromal, needle-like, branching, white-grey crystalline infiltrate extending peripherally is highly characteristic (Fig 1, Fig 2) [6] . Some studies have described this characteristic pattern as snowflake in appearance. Although classically resulting in anterior to mid stromal crystalline deposits, cases of predominantly posterior stromal involvement have been reported [7]. The overlying epithelium is typically intact. The anterior chamber is generally quiet with only rare cell, however cases with hypopyon have also been reported [6].
Differential Diagnosis
- Bacterial keratitis
- Viral keratitis
- Fungal keratitis
- Cystinosis
- Bietti crystalline corneoretinal dystrophy
- Schnyder crystalline corneal dystrophy
- Multiple Myeloma
- Monoclonal gammopathy
- Tyrosinemia
- Medication induced- Fluoroquinolones [3][8]
Diagnosis & Diagnostic Procedures
Culturing of the cornea in cases of suspected ICK can be utilized in the diagnosis and confirmation of infectious crystalline keratopathy. Unfortunately, due to the presence of an intact epithelium in many cases, simple corneal scrapings may be inadequate. Diagnostic keratectomy of the affected cornea with cultures and cytology has high yield for identifying the underlying organism. For cases that are refractory to medical management requiring penetrating keratoplasty, histologic examination of the removed corneal graft can confirm the diagnosis. Conjunctival swabs are not adequate for diagnosis[6].
General Pathology and Histology
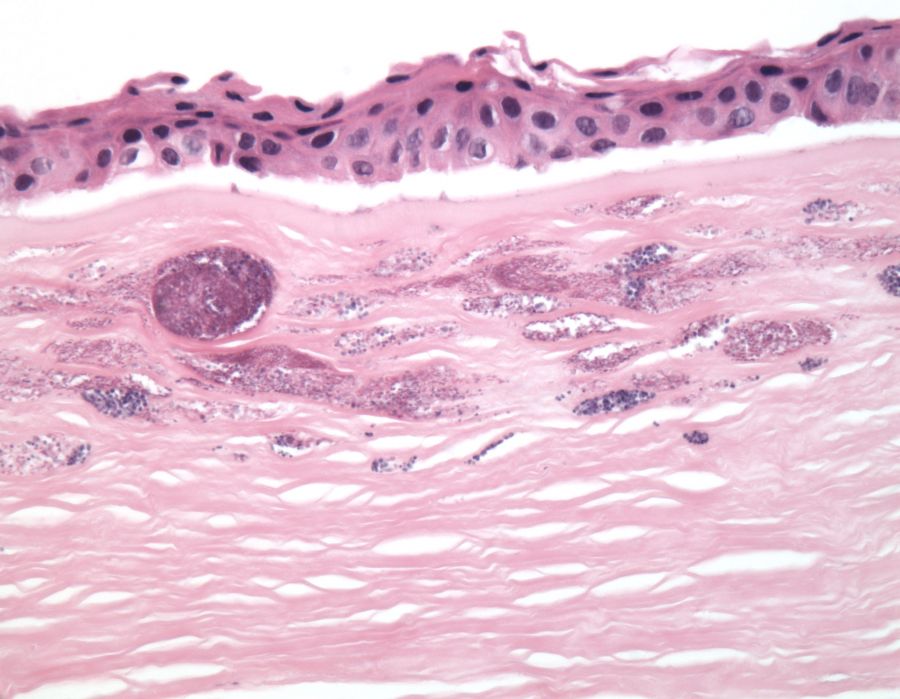
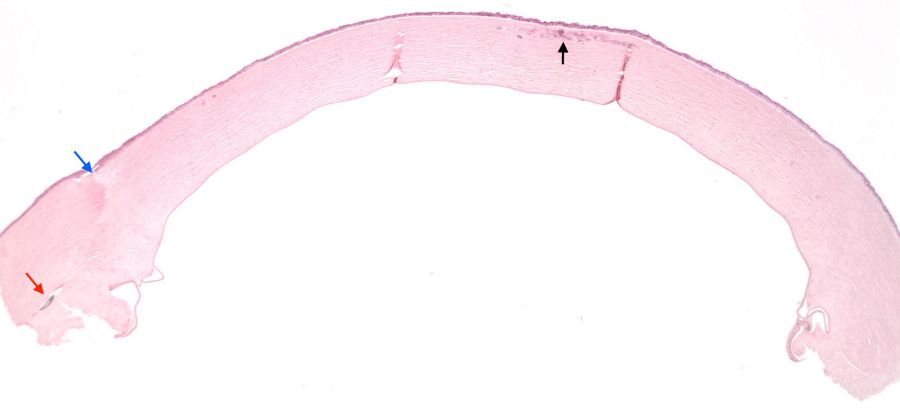
Histopathologic examination of affected corneal buttons have classically demonstrated gram positive cocci in the void spaces between collagen lamellae of the anterior stroma with little surrounding inflammatory infiltrate (Fig 3, Fig 4). Although gram positive cocci, most commonly streptococcus viridans, have been associated with the development of ICK, other bacterial and fungal organisms have also been observed. However, regardless of the causative infectious organism, the histology of ICK specimens is characterized by an absence of inflammatory infiltrate surrounding the infectious organisms. Electron microscopy of the infected cornea can also be performed, helping identify the presence of an underlying organism[2][6].
General treatment & Management
Infectious crystalline keratopathy can be profoundly difficult to treat. In many cases, ICK can remain refractory to medical treatment alone and surgical excision with a penetrating keratoplasty may be required to clear the infection. The tenacity of these infections is thought to be related to the secretion of a biofilm by the offending organism. The presence of biofilm is theorized to protect the underlying pathogen from both topical and systemic antibiotics[9]. Treatment often involves discontinuation or a taper in steroid drops to alleviate the immunocompromised state of the cornea and to recruit the host immune system. Aggressive antibiotic regimens, including the use of fluoroquinolones, cephalosporins, and vancomycin have all been employed in the treatment of ICK with varying results. A recent publication by Tu, et al. demonstrated significant efficacy in the management of ICK with use of topical linezolid 0.2%. The increased efficacy of topical linezolid over vancomycin may be due to increased tolerability of the medication as well as improved biofilm penetration[10][11]. Reports of growing antimicrobial resistance among infectious pathogens associated with ocular infections like ICK further demonstrates the need for additional treatment options [12].
References
- ↑ 1.0 1.1 Bowling, Brad. Kanski’s Clinical Ophthalmology A Systemic Approach. Eighth Edition. Elsevier Edinburgh;2016.
- ↑ 2.0 2.1 2.2 2.3 Mannis MJ, Holland EJ. Cornea, Surgery of the Cornea and Conjunctiva. Fourth Edition. Elsevier 2017.
- ↑ 3.0 3.1 Weisenthal RW, Afshari NA, Bouchard CS, Colby KA, Rootman DS, Tu EY, de Freitas D. BCSC External Disease and Cornea, Section 8. American Academy of Ophthalmology;2016.
- ↑ 4.0 4.1 Gorovoy MS, Stern GA, Hood Cl, Allen C. Intrastromal noninflammatory bacterial colonization of a corneal graft. Arch Ophthalmol. 1983;101(11):1749-52.
- ↑ Meisler DM, Langston RH, Naab TJ, Aaby AA, McMahon JT, Tubbs RR. Infectious crystalline keratopathy. Am J Ophthalmol. 1984; 97(3):337-43.
- ↑ 6.0 6.1 6.2 6.3 6.4 Kinota S, Wong KW, Biswas J, Rao NA. Changing patterns of infectious keratitis: overview of clinical and histopathologic features of keratitis due to acanthamoeba or atypical mycobacteria, and of infectious crystalline keratopathy. Indian J Ophthalmol. 1993; 41(1):3-14.
- ↑ Mesiwala NK, Chu CT, Raju LV. Infectious crystalline keratopathy predominantly affecting the posterior cornea. Int J Clin Exp Pathol. 2014;7(8):5250-3. eCollection 2014.
- ↑ Gupta PK, Kharod BV, Afshari NA. Crystalline Keratopathy: Spectrum of Disease, Diagnosis and Treatment. AAO.Org. https://www.aao.org/eyenet/article/crystalline-keratopathy-spectrum-of-disease-diagno. September 1st, 2017.
- ↑ Fulcher TP, Dart JK, McLaughlin-Borlace L, Howes R, Matheson M, Cree I. Demonstration of biofilm in infectious crystalline keratopathy using ruthenium red and electron microscopy. Ophthalmology.2001;108(6):1088-92.
- ↑ Tu EY, Jain S. Topical linezolid 0.2% for the treatment of vancomycin-resistant or vancomycin-intolerant gram-positive bacterial keratitis. Am J Ophthalmol. 2013;155(6):1095-1098.e1. doi: 10.1016/j.ajo.2013.01.010. Pub 2013 Feb 26.
- ↑ Farooq AV, Hou JH, Jassim S, Hag Z, Tu EY, de la Cruz J, Cortina MS. Biofilm Formation on Bandage Contact Lenses Worn by Patients with the Boston Type 1 Keratoprosthesis: A Pilot Comparison Study of Prophylactic Topical Vancomycin 15 mg/mL and Linezolid 0.2. Eye Contact Lens. 2016 Oct. [Epub ahead of print]
- ↑ Haas W, Pillar CM, Torres M, Morris TW, Sahm DF. Monitoring antibiotic resistance in ocular microorganisms: results from the Antibiotic Resistance Monitoring in Ocular micRorganisms (ARMOR) 2009 surveillance study. Am J Ophthalmol. 2011;152(4):567-574.e3. doi: 10.1016/j.ajo.2011.03.010. Epub 2011.