Inborn Errors of Galactose Metabolism
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
Disease
Inborn errors of galactose metabolism, also known as galactosemias, include any disorder that interferes with galactose metabolism, leading to the accumulation of metabolites, mainly galactitol, responsible for syndromic symptoms. These congenital diseases typically present within the first year of life, though vary in severity and thus may be asymptomatic. In the eye, they have varying degrees of association with cataract formation, thus are necessary to understand from an ophthalmologic perspective. The enzymes that, to date, have been identified to cause inborn errors of galactose metabolism are: galactose 1-P uridyltransferase (GALT), galactokinase (GALK), UDP-galactose 4’ epimerase (GALE) and galactose mutarotase deficiency (GALM). The overall pathophysiology of galactosemias, followed by a breakdown of each of the subtypes will be reviewed in further detail below.
Pathophysiology
Galactosemias are due to deficiencies of enzymes responsible for galactose metabolism. To better understand the pathophysiology of these diseases, it is necessary to understand the biochemistry of galactose metabolism.
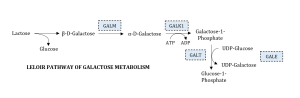
Galactose is metabolized through the Leloir pathway. There are 4 major cytosolic enzymes in galactose metabolism, and each, when deficient, is responsible for producing a variant of galactosemia. Specifically, these enzymes are: 1) galactose mutarotase (GALM), 2) galactokinase (GALK1), 3) galactose-1-phosphate uridyltransferase (GALT) and 4) UDP-galactose 4’-epimerase (GALE).
Under normal conditions, the body obtains galactose from either endogenous or exogenous sources. Once obtained, galactose is converted from its β-D-galactose to its α-D-galactose conformation by galactose mutarotase. Then, it is is phosphorylated to galactose-1-phosphate by galactokinase (GALK). Next, galactose-1-phosphate uridyltransferase (GALT) catalyzes the displacement of galactose-1-phosphate with UDP-glucose, forming glucose-1-phosphate and UDP-galactose. Finally, UDP-glucose is recycled back to UDP-galactose by galactose-4’-epimerase (GALE) [Figure 1].
When enzymatic mutations prevent the Leloir pathway from functioning properly, metabolites upstream of the mutation accumulate and are shunted to alternative pathways of metabolism. The main alternative pathway of interest in ophthalmology is the Aldolase Reductase pathway, as it generates galactitol, which is responsible for causing cataract formation in certain galactosemias. This is because galactitol is not only osmotically active but also tends to accumulate in the lens fiber cells, due to the high concentration of aldolase reductase at the anterior side of the lens.[1] This accumulation can lead to lens swelling, cell lysis, and ultimately cataract formation.[1]
Subtypes
Nuclear cataract formation is a common feature across most forms of galactosemia, except for peripheral GALE deficiencies and Duarte Galactosemia, as described below. By managing galactose intake and monitoring for ophthalmological changes, potential complications related to cataracts can be minimized.
- Type I - Classic Galactosemia (GALT) – This autosomal recessive metabolic syndrome typically presents within a newborn’s first week of life with any or all the following: liver failure, jaundice, cataracts, Escherichia coli sepsis, hypoglycemia, bleeding diathesis, poor weight gain, seizures, renal failure, cerebral edema, neutropenia.[2][3] Therefore, this form is included in newborn screening programs, as prompt galactose restriction is necessary for vitality.[2] Long-term outcomes in classic galactosemia can be associated with a variety of symptoms including speech delays and learning disabilities, impaired motor function and balance, and ovarian failure.[3] Cataract formation even in the absence of dietary restriction of dairy products is still possible.[3]
- Duarte Galactosemia (GALT – variation) – This is an allelic variant of classic galactosemia, in which there is partial impairment of the GALT enzyme. Since individuals with Duarte galactosemia can still metabolize galactose, the systemic manifestations are less severe than classic galactosemia. In addition, it is not associated with cataract formation.[4]
- Type II – Galactokinase Deficiency (GALK) – This mutation presents insidiously due to its milder subtype and is most associated with the manifestation of cataracts. It is discussed in depth here; briefly, it is associated with infantile and presenile cataract. When treatment is started within two to three weeks of age, reversal of cataract formation can occur.
- Type III - Uridine Diphosphate Galactose-4-Epimerase Deficiency (GALE) – This mutation encompasses a spectrum of disease activity, broadly categorized into "central" and "peripheral" forms.[2] Central disease represents the most severe manifestation, manifesting similarly to classic galactosemia.[3] This form requires immediate restriction of galactose and lactose in the diet. Specifically, some symptoms seen in the central form of this disease are: hypotonia, poor feeding, vomiting, weight loss, jaundice, hepatomegaly, liver dysfunction, aminoaciduria, and cataract formation.[2]
- In contrast, peripheral disease is relatively mild, primarily affecting erythrocytes and leukocytes.[5] Dietary supplementation is not typically necessary for individuals with this form of the disease. Moreover, peripheral GALE deficiency is not associated with cataract formation, which eliminates the need for additional ophthalmological evaluation.[6]
- Type IV - Galactose mutarotase deficiency (GALM) – This form of galactosemia is a relatively recent addition to the spectrum of galactosemia disorders. It shares similarities with GALK deficiency in terms of clinical presentation.As it is a more recently identified form, further research is needed to fully understand the range of symptoms and complications associated with GALM.[7]
Diagnosis
Differential diagnosis
As the main ophthalmologic effect of galactosemia is the development of cataracts, the differential diagnosis includes any other cause of cataracts that correlates with the patient’s age. For a more complete description of cataract work-up, visit this article on pediatric cataracts.
Management
General treatment
As described above, the mainstay treatment for symptomatic forms of galactosemias is galactose restriction, primarily in the form of dairy restriction.
Despite the introduction of a galactose-restricted diet, there is still a possibility of developing cataracts; however, it is uncertain whether this is due to lack of adherence to the diet or an alternative pathological mechanism. Age-specified cataract management is warranted when visually significant impairment exists.[8]
Follow-Up
Long-term follow-ups with the patients’ primary care provider are necessary to monitor their measurements of galactose, its derivative metabolites, and measurements of galactitol in red blood cells,[9][10] as well as monitor for systemic symptoms associated with these conditions. Routine ophthalmologic evaluations are also necessary to evaluate for cataract formation.
References
- ↑ Jump up to: 1.0 1.1 Ai Y, Zheng Z, O’Brien-Jenkins A, et al. A mouse model of galactose-induced cataracts. Hum Mol Genet. 2000;9(12):1821-1827. doi:10.1093/hmg/9.12.1821
- ↑ Jump up to: 2.0 2.1 2.2 2.3 Lak R, Yazdizadeh B, Davari M, Nouhi M, Kelishadi R. Newborn screening for galactosaemia. Cochrane Cystic Fibrosis and Genetic Disorders Group, ed. Cochrane Database Syst Rev. Published online December 23, 2017. doi:10.1002/14651858.CD012272.pub2
- ↑ Jump up to: 3.0 3.1 3.2 3.3 Kishnani PS. Glycogen Storage Diseases and Other Inherited Disorders of Carbohydrate Metabolism. In: Loscalzo J, Fauci A, Kasper D, Hauser S, Longo D, Jameson JL, eds. Harrison’s Principles of Internal Medicine, 21e. McGraw-Hill Education; 2022. Accessed July 10, 2023. accessmedicine.mhmedical.com/content.aspx?aid=1190503420
- ↑ Nema N, Kumar R, Verma A, Verma S, Chaturvedi K. Association of presenile cataract with galactose-1-phosphate uridyl transferase gene mutations. Natl Med J India. 2017;30(2):73-75.
- ↑ Fridovich-Keil J, Bean L, He M, Schroer R. Epimerase Deficiency Galactosemia. In: Adam MP, Mirzaa GM, Pagon RA, et al., eds. GeneReviews®. University of Washington, Seattle; 1993. Accessed July 9, 2023. http://www.ncbi.nlm.nih.gov/books/NBK51671/
- ↑ Fridovich-Keil J, Bean L, He M, Schroer R. Epimerase Deficiency Galactosemia. In: Adam MP, Mirzaa GM, Pagon RA, et al., eds. GeneReviews®. University of Washington, Seattle; 1993. Accessed July 9, 2023. http://www.ncbi.nlm.nih.gov/books/NBK51671/
- ↑ Timson DJ. Type IV galactosemia. Genet Med. 2019;21(6):1283-1285. doi:10.1038/s41436-018-0359-z
- ↑ Rubio-Gozalbo ME, Derks B, Das AM, et al. Galactokinase deficiency: lessons from the GalNet registry. Genet Med Off J Am Coll Med Genet. 2021;23(1):202-210. doi:10.1038/s41436-020-00942-9
- ↑ Los E, Ford GA. Galactose-1-Phosphate Uridyltransferase Deficiency. In: StatPearls. StatPearls Publishing; 2023. Accessed July 9, 2023. http://www.ncbi.nlm.nih.gov/books/NBK441957/
- ↑ Ramani PK, Arya K. Galactokinase Deficiency. In: StatPearls. StatPearls Publishing; 2023. Accessed July 9, 2023. http://www.ncbi.nlm.nih.gov/books/NBK560683/