Imaging in Ocular and Ocular Adnexal Trauma
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
Background
Globe injuries are one of the important causes of visual disability. The World Health Organization estimates that eye injuries result in blindness in about 1.6 million people and unilateral blindness or decreased vision in almost 19 million people each year. The overall prevalence of trauma-related eye injuries is approximately 2%–6%, with as many as 97% of cases resulting from blunt trauma.[1]
Patients with facial fractures related to trauma are at an increased risk for associated eye injuries, and the incidence of vision loss and blindness related to facial fractures may be as high as 10.8%. Ocular involvement may also be seen in as many as 84% of patients with head injuries.[2] The presence of surrounding periorbital soft-tissue swelling and other associated injuries, physical examination of the globe may be challenging during acute trauma, along with difficulties related to the patient cooperation caused by sedation, unresponsiveness, altered state of mind. In the presence of such factors, the extent of injuries can be assessed by imaging techniques. Various imaging techniques, which are used in ocular adnexal trauma as follows:
- Ultrasonography (USG)
- Ultrasound Biomicroscopy (UBM)
- Optical coherence tomography (OCT)
- Fundus Fluorescein Angiography (FFA)
- Indocyanine Green Angiography (ICG)
- Fundus Auto Fluorescence (FAF)
- Plain x-ray
- Computerized Tomography (CT scan)
- Magnetic Resonance Imaging (MRI)
In general, conventional A & B scan ultrasonography, high frequency ultrasound Biomicroscopy, Optical coherence tomography, Fundus fluorescein and Indocyanine Green Angiography and Fundus autofluorescence are useful in the diagnosis and monitoring intraocular injuries. Plan x-ray, computerized tomography (CT) and Magnetic Resonance Imaging (MRI) are employed primarily for orbit, optic nerve and ocular adnexal injuries.
Imaging
Ultrasonography
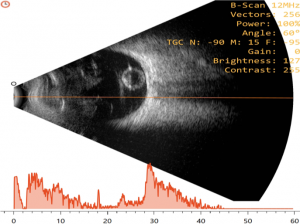
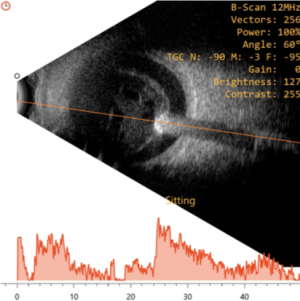
Conventional ophthalmic ultrasonography (USG) uses a probe that oscillates between 7.5 – 12 Mega Hertz. Diagnostic ophthalmic ultrasonography may employ either a unidirectional amplitude modulated (A scan), first reported in 1956 by Mundt and Hughes and the two-directional cross-sectional brightness (B scan) scan, introduced in ophthalmic practice by Baum and Greenwood in 1958. Frequently, both the A-scan and B-scan complement each other and are often used together.It is non-invasive, non-ionizing, inexpensive, and easily performed imaging technique. It is useful in detecting intraocular foreign bodies (IOFB) and outlining soft tissue abnormalities of the eye and orbit in presence of media opacity.[3] It is relatively contraindicated in the presence of open globe injury, to avoid further trauma and wound contamination. Thus, in case of a ruptured globe, it is highly recommended that primary closure should be performed prior to ultrasound examination. If ultrasound examination is performed prior to primary closure, it should be performed with caution to minimize possible trauma to the eye. Sterility is essential if the globe is open or a wound has been recently closed. The probes should be sterile, or they may be placed in sterile rubber sleeves.[4]
Anterior chamber
Ocular trauma with hyphema and corneal opacities may obscure visualization of anterior segment and require ultrasound evaluation. Ultrasound may reveal the presence of shallow anterior chamber, gross angle recession and ciliary body detachment. In the presence of hyphema, ultrasonography may reveal multiple echoes in the anterior chamber. Angle recession is suspected if the anterior chamber is deep with wide angles. Foreign bodies or evidence of anterior segment structure abnormalities may be detected using immersion or water balloon technique. More recently, ultrasound biomicroscopy has been introduced for more detailed evaluation of the anterior segment.[4]
Lens
The presence, position and integrity of the lens should be assessed following ocular trauma. Anterior or posterior capsule may be ruptured in ocular trauma. The detection of lens rupture may be useful in determining the appropriate surgical technique for cataract extraction. Vitreo-retinal abnormalities have been reported to be associated with traumatic cataract as high as 20-30%5. Subluxation or dislocations of the lens also can be easily detected with USG B scan. [5] Posterior segment structures that may be imaged in the presence of anterior segment opacity include the vitreous, retina and choroid. Common posterior segment conditions following blunt and penetrating injuries that may be diagnosed using ultrasound B-scan include the following: posterior vitreous detachment, vitreous hemorrhage, retinal breaks, retinal detachment, retinal dialysis, choroidal rupture, choroidal detachment, and suprachoroidal hemorrhage.
Vitreous
Vitreous hemorrhage is one of the commonest findings in patients with trauma. Recent mild hemorrhage is imaged as dots and short lines on B-scan, and as chain of low amplitude spikes on A-scan. With dense hemorrhage, more opacity is seen on B-scan and higher is their reflectivity on A-scan. Posterior vitreous detachment (PVD) is usually smooth and may be thick when blood is layered along its surfaces on USG B-scan. Reflectivity may vary from extremely low (as in the normal eyes) to extremely high (as with dense hemorrhage). Kinetic B-scan ultrasonography typically shows an undulating after-movement on B-scan, which normally allows the PVD to be differentiated from less mobile retinal and choroidal detachments.[4]On A-scan, horizontal and vertical spikes after movement are seen. However, there are situations in which the distinction of PVD from retinal detachment may be quite challenging. Vitreous traction bands demonstrated ultrasonically may be an indirect sign of vitreous incarceration within a perforation site. These bands may help identify sites of scleral rupture while the opposite may be the site of retinal breaks and detachment.[4]
Retina and Choroid
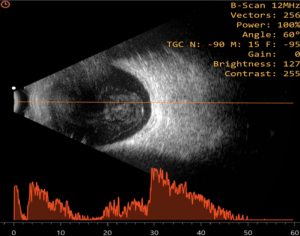
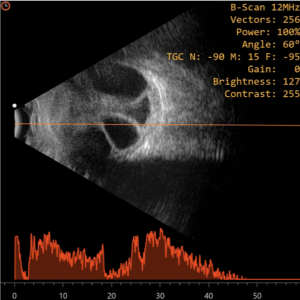
Retinal detachment (RD) - rhegmatogenous or tractional, may occur following ocular trauma and can be diagnosed by ultrasound in the presence of opaque media. Traction can occur within the vitreous base either near the injury site or at the opposite quadrant. Rhegmatogenous RD typically appears as a bright, continuous, smooth and somewhat folded membrane within the vitreous, which is reflective and freely mobile on real time imaging. In cases of total or extensive retinal detachment, the detached retina has a typical triangular shape with insertion into the optic disc and ora serrata. On A-scan, a 100% tall spike is produced when the sound beam is directed perpendicular to the detached retina. The traction pattern appears as a tented or tabletop configuration with the vitreous band connected to the anterior surface.[6] Peripheral retinal tears and retinal dialysis may also occur in blunt trauma. This can be detected on longitudinal B Scan by noting the disinsertion of the peripheral retina from the ora serrata. Retinal dialysis, when present, is most frequently seen in the upper nasal quadrant.[6]
Choroidal
Choroidal detachment on B-scan typically appears as a smooth, thick, dome-shaped membrane in the periphery with little after-movement on kinetic evaluation. On A-scan, at tissue sensitivity, a thick, steeply rising 100% high spike is produced, which on low sensitivity, appears as a double peaked spike. On kinetic A Scan, Slight vertical after-movement is normally appreciated. When 360-degree choroidal detachment is present and imaged with a transverse B-scan, multiple bullae produce a scalloped appearance and named ‘kissing choroidals’[6]
In cases of massive suprachoroidal hemorrhage where the detachments are highly elevated, ultrasound follow-up is of utmost importance in evaluating clot size and appearance which help to determine the threshold and timing for surgical intervention [6]
Sclera
Blunt trauma can lead to posterior scleral rupture that might be difficult to detect clinically. USG can show the area of irregular contours and decreased reflectivity at the site of scleral rupture. A scleral rupture may be suspected when some of the following indirect signs exist:
- Incarceration of vitreous and vitreous hemorrhage with PVD
- Demonstration of traction bands or folds that extend in the direction of rupture
- Thickening or detachment of retina or choroid
- Episcleral hemorrhage closest to the site of disruption.
Intraocular foreign body
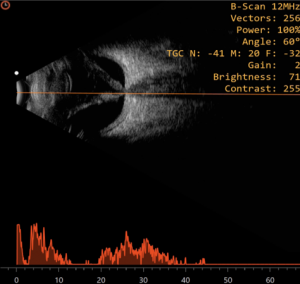
Ultrasound B-Scan is a complementary modality to radiology in the detection and localization of foreign bodies. It provides additional information regarding the exact location within the eye and the extent of damage to the surrounding tissues. Softer materials like wood and other vegetative material have intermediate reflectivities which are more difficult to detect. Small particles particularly located at the orbital apex and those lodged in highly reflective tissues can often be missed. If the foreign body is not detectable clearly due to echoes from nearby tissues, reducing the amplitude gain helps confirm the same against less reflective tissues.[4]
On ultrasound A-scan, IOFBs are seen as a steeply rising wide echo spike, noted along the baseline between the initial spike and ocular wall spike. The reflectivity of the lesion spike is extremely high (100%) which persists on low gain with sound attenuation. The distance between the IOFB and the adjacent sclera is accurately measured at lower system sensitivity with sound attenuation.
On ultrasound B-scan, IOFBs appear acoustically opaque contrasting with the acoustically clear vitreous and remains displayed even when the system sensitivity is decreased by 20-30 db.[6] These foreign bodies can be precisely localized to the different quadrants and the distance of foreign body to adjacent intraocular tissues can be assessed. Mobility of the IOFB can be also be assessed with topographic and kinetic echography which can help identify if the foreign body is adherent to the retina or floating in the vitreous. Furthermore, as glass and metallic foreign bodies are highly reflective, sound attenuation is very strong with shadowing of the posterior ocular and orbital tissues.[7] As noted above, additional benefits of B-scan ultrasonography in the setting of an IOFB is the ability to detect associated intraocular pathology like vitreous hemorrhage, vitreous bands, fibrosis, retinal detachment, choroidal detachment and even scleral entry wounds.[3]
Ultrasound Biomicroscopy
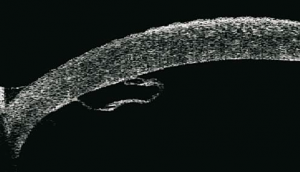
The inability to visualize the anterior segment by conventional ultrasound B-Scan and high resolution radiologic images led to a shift towards alternate imaging modalities. The first practical Ultrasound biomicroscopy (UBM) system was developed by Foster and Pavlin in the early 1990s. UBM is an imaging technique that uses high-frequency (35-50MHz) sound waves to produce high-resolution, cross-sectional images of the anterior segment to a depth of approximately 5 mm.[8] Thus UBM is suitable for detailed imaging of anterior segment anatomy and pathology, including the cornea, irido-corneal angle, anterior chamber, iris, ciliary body and lens.[3]
UBM also has a great value in the presence of corneal pathology such as edema, keratoconus or corneal scars. It can demonstrate disruption of the intraocular structures as seen in iridodialysis, angle recession, cyclodialysis, zonular rupture, scleral laceration, foreign body and epithelial ingrowth, irrespective of the opaque media, and can provide evidence for treatment.[3]
An additional advantage of UBM is that images are acquired in real time with mobility of various structures visualized in situ. High frequency (50 MHZ) ultrasound also identify the exact loation, size, and nature of a foreign body along with the appearance of surrounding tissue much better than conventional low frequency (10 MHZ) ultrasound.[9] Although CT is superior for detecting foreign bodies, ultrasound is more useful for localizing foreign bodies relative to the ocular coats. It is also superior to CT in showing extent of ocular damage associated with an IOFB. UBM is also useful for detection and localization of small superficial non-metallic foreign bodies that are usually missed on CT and conventional USG.[10] UBM can be used to accurately assess the zonules. Zonular defects diagnosed by UBM in cases of traumatic cataract allows the ophthalmic surgeon to plan preoperatively to prevent vitreous loss or nucleus drop in the vitreous cavity [11]
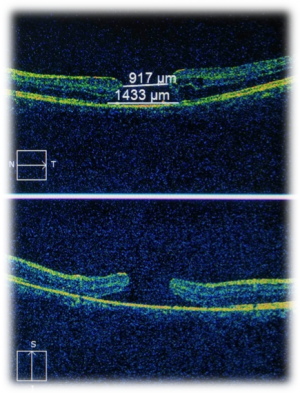
Optical coherence tomography (OCT)
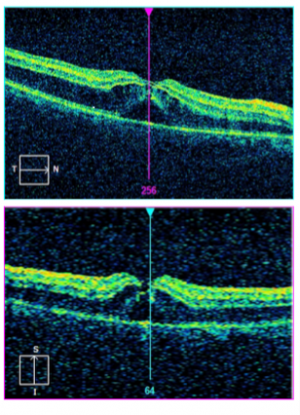
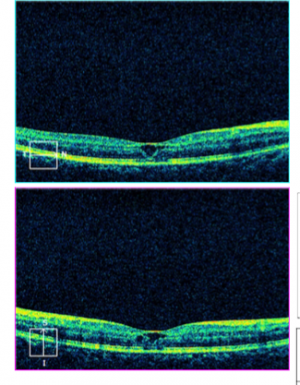
Optical coherence tomography (OCT) is a non- contact, high-resolution and cross-sectional imaging modality that utilizes low coherence interferometry principle. It can achieve axial resolution in the range of 3–20 μm.[12]Posterior segment OCT (PSOCT) uses a lower wavelength of light at 830 nm while Anterior Segment OCT (ASOCT) uses a higher wavelength 1310 nm.
Anterior Segment Optical Coherence Tomography (ASOCT)
In ocular trauma, ASOCT helps to support the diagnosis of ocular surface injuries and intraocular lesions. It has advantages of accuracy, repeatability and can be performed in penetrating ocular injuries as it is non-contact imaging technique. ASOCT can be used to visualize Descemet’s membrane detachment or angle closure in the presence of hazy cornea. Other conditions that may be diagnosed with ASOCT include corneal intrastromal foreign bodies, its depth, up to 6mm [2][13],localization and presence of residual foreign bodies, damage to the angle structures and the presence of cyclodialysis cleft.
In clinical situations where gonioscopy can be quite challenging such as in the presence of hypotony from cyclodialysis clefts resulting in a shallow anterior chamber precluding visualization of the iridocorneal angle, ASOCT may be a great alternative or adjunctive modality to evaluate the angle anatomy.[14]
Limitations of ASOCT however include inability to penetrate pigmented tissue and inability to image past the posterior pigmented epithelium of the iris.
Posterior segment Optical Coherence Tomography (PSOCT)
In ocular trauma, posterior segment OCT may aid in diagnosing and assessing Berlin’s edema [15], macular hole[16] [17], preretinal or submacular hemorrhage, retinal detachment, direct or indirect choroidal rupture [18] [19] ,choroidal detachment, retinal pigment epithelial tears [20], optic disc pit or traumatic retinoschisis. It is easy, quick and reliable tool for diagnosis and monitor macular and chorioretinal disease with high accuracy and precision.
Fluorescein Angiography
Fluorescein Angiography (FFA) is a commonly performed study useful in the diagnosis, management and monitoring of posterior segment retinal and choroidal abnormalities. Angiographic findings may vary from normal to hypo to hypofluorescence depending on the underlying pathology. In cases of severe trauma, fluorescein leakage is observed denoting disruption of the RPE outer blood retinal barrier. [21] In mild injuries there may be no breakdown of the blood retinal barrier with just disruption of the outer segments of photoreceptors. In late Retinal Pigment Epithelium (RPE) involvement with atrophic, these may present as window defects.[22] In cases of sympathetic ophthalmia, fluorescein angiographic may feature starry, pin point leakages in the sympathizing eye. [23]
Areas of retinal opacity with fluorescein leakage following trauma may develop a salt-and-pepper appearance which correlate with focal loss of retinal function giving rise to scotomas.[21] [24] FFA is thus a very useful modality not only in diagnosing but also prognosticating in ocular trauma involving the posterior segment of the eye.
Indocyanine Green Angiography
Indocyanine Green Angiography (ICG) is an excellent diagnostic tool to evaluate choroidal pathologies. Features such as local delayed filling along the choroidal vessels and delayed filling of choroidal veins are classic signs of choroidal insult. There might also be intrachoroidal ICG leakage around vessels, including vortex veins which indicates damage to the veins draining the choroid.[25]
Most often, dilation of small choroidal vessels and narrowing and irregular diameters of choriocapillaris can also be observed at the indocyanine green leakage site, which often coincides with the fluorescein leakage. It has been postulated that if such ICG findings are present, they may not only indicate breakdown of the outer blood-retinal barrier but also increased permeability of the choriocapillaris due to trauma. ICG can also help in identifying CNV following traumatic choroidal ruptures.[26]
The choroidal circulation is vital to the outer retinal layer; hence any typical ICG findings as mentioned above may also play a role in the prognosis for visual recovery in these cases.
Fundus autofluorescence
Fundus autofluorescence (FAF) imaging is a non-invasive method for assessing changes in the posterior segment of the eye resulting from blunt ocular trauma. It is a very helpful in-vivo mapping of naturally or pathologically occurring fluorophores of the ocular fundus. The dominant sources are fluorophores accumulating in lipofuscin (LF) granules in the retinal pigment epithelium (RPE). In the absence of RPE cells, minor fluorophores including collagen and elastin, e.g., in choroidal blood vessel walls, may also become visible. Bleaching phenomena and loss of photopigment may result in increased FAF by reduced absorbance anterior to the RPE level [27].
As normal photoreceptor function is dependent on normal RPE function, any imbalance following ocular trauma leads to accumulation of the toxic fluorophores and byproducts and eventually cell death.[28] Using this property, it is known that the damaged RPE areas are more evident and better delineated by FAF imaging compared with fundus examination and fundus photography alone.
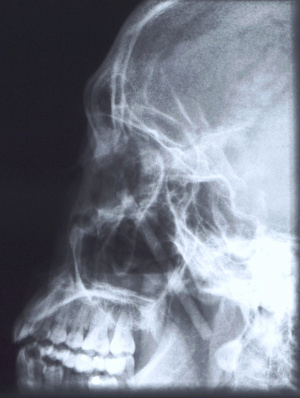
x-ray
Plain x-rays have a limited role in ophthalmic trauma in modern times. Historically it was the primary imaging of choice for the diagnosis of orbital and orbitofacial fractures. However, owing to the high degree of false negatives, and occasional false positives they are no longer utilized for that indication or in isolation. They are currently performed as a screening tool for radiopaque foreign bodies of the orbits and face. Prior to the advent of high-resolution CT scans, x-rays were used as a localizing tool prior to intervention for intraocular foreign bodies. This was achieved by obtaining images in various positions of gaze in which the foreign body could be seen moving within or against the planet of movement of the globe.
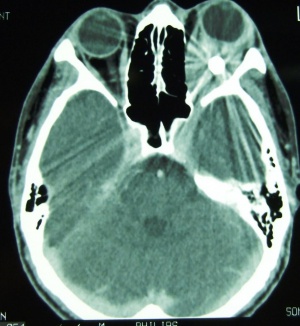
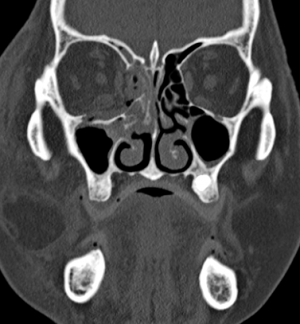
Computerized tomography (CT scan)
Computerized tomography is the imaging of choice for evaluating suspected orbital and orbitofacial fractures, intraorbital and intraocular foreign bodies and traumatic optic neuropathies. In the trauma setting, it is generally performed as a non-contrast study, with contrast indicated only in cases where there is concern for carotid-cavernous fistula. Axial images are useful in evaluation of the medial and lateral orbital walls, in addition to the medial and lateral rectus muscles. Coronal images are similarly useful in scrutinizing the superior and inferior orbital walls, the superior and inferior rectus muscles, and identifying optic nerve sheath hematoma. [29] While soft tissue window is useful for gross disruptions of the bone and soft tissue changes (EOM, Fat, Optic nerve, Brain, etc), Bone Windows are much more sensitive to detect orbital and orbitofacial fractures and should be viewed in the axial, coronal and sagittal views.
In the setting of orbital wall fracture, CT scan can often be helpful in demonstrating entrapped extraocular muscles. CT typically demonstrates linear floor fractures with minimal displacement and little or no soft tissue displacement in the maxillary antrum. However, associated soft tissue swelling, fat stranding, and muscular hematoma can often make radiographic interpretation difficult, especially in children where CT findings of entrapped extraocular tissues can be minimal. This emphasizes the importance of clinical exam using assessment of extraocular motility and forced ductions. Finally, CT can also be used o estimate the fracture area, which can be utilized as criterion for operative repair. [30]
In situations where a detailed clinical examination is not possible and there is a high suspicion of globe disruption, a CT scan may be indicated.[31] In patients with a clinical diagnosis of traumatic optic neuropathy, CT can be employed to asses for optic nerve canal fracture, foreign body, or bony impingment on the nerve.[32] It can also be employed for intraoperative navigation of the orbitofacial skeleton with 3-D modeling, in which images are acquired in the 0.6 – 1 mm formats. 3D reconstructions may be performed for patient education and treatment planning, which may be used for prebending orbital implants.
CT can also aid in the diagnosis of “open globe” injuries, defined as a full-thickness disruption of the sclera or cornea of the eye. Findings suggestive of an open globe injury on CT include a disruption of the globe contour, change in globe volume, variation in anterior chamber depth, and finally intraocular air, blood or foreign body. In one study, the sensitivity and specificity of detecting open globe injuries with CT was estimated at 75% and 93%, respectively. Therefore, although useful in the evaluation of traumatic injury to the globe, CT should not be relied upon solely for the assessment of open globe injuries. [29] Finally, CT scans of the orbits and face and not infrequently to assess completeness and accuracy of orbital reconstruction following fracture repairs, especially when postoperative complicaitons such as strabismus, motility disorders or optic neuropathy.
Cone Beam CT scans (CBCTs) may be considered in special situations either as a screening tool for bony injuries or as a postoperative evaluative tool following orbitofacial trauma repair especially with radiopaque implants.
Magnetic resonance imaging (MRI)
Magnetic resonance imaging has a limited role in ocular and ocular adnexal trauma and hence rarely indicated in the acute posttraumatic situation. It is also contraindicated unless metallic magnetizable foreign bodies of the face, orbits and elsewhere in the body have been definitively ruled out using CT, Xray, or ultrasound. While CT scans may provide most of the information required to support a clinical diagnosis of an open or closed globe injury, intraocular or intraorbital foreign bodies or optic canal fractures etc, MRI may be indicated in patients with suspected optic nerve or optic nerve sheath hematoma and traumatic carotico-cavernous fistula. [33] Suspected organic intraocular foreign bodies not imaged on CT can sometimes be visualized on MRI. However, the characteristics of organic bodies is highly variable with many demonstrating similar intensity to air, bone fragments, or blood. Therefore, the diagnostic use of MRI in evaluating foreign bodies and differentiating from native structures remains limited.[33]
In summary, a comprehensive knowledge of various ocular and ocular adnexal imaging techniques, with judicious and appropriate use of the investigations techniques, relative and absolute contraindications coupled with interpretation, aids developing a holistic diagnosis of structural abnormalities. This in turn guides the physician not only in making a more complete diagnosis, but also aids in patient counseling followed by appropriate investigation and management, which helps deliver the most appropriate care for the patient.
Conclusion
In summary, apart from good history elicitation , thorough, atraumatic and painless ophthalmic and orbitofacial examination, appropriate use of imaging helps formulate a complete diagnosis, facilitating patient counselling, treatment planning and monitoring the patients postoperatively
References
- ↑ Koo L, Kapadia MK, Singh RP, Sheridan R, Hatton MP. Gender differences in etiology and outcome of open globe injuries. J Trauma 2005; 59(1):175–178.
- ↑ Jump up to: 2.0 2.1 Guly CM, Guly HR, Bouamra O, Gray RH, Lecky FE. Ocular injuries in patients with major trauma. Emerg Med J 2006; 23(12):915–917.
- ↑ Jump up to: 3.0 3.1 3.2 3.3 S Gupta, G Kaur, VS Gurunadh, MA Khan. Evolution: Evolution of Imaging Modalities in Ocular Trauma. DOS Times - Vol. 20, No. 8 February, 2015.
- ↑ Jump up to: 4.0 4.1 4.2 4.3 4.4 Susheel,Chugh JP, Verma M. Role of ultrasonography in ocular trauma. Indian Journal of Radiology and Imaging, Vol. 11, No. 2, April-June, 2001, pp. 75-79
- ↑ Kaskalogu M. US findings in eyes with traumatic cataracts. Am J Ophthalmol 1985; 9: 496
- ↑ Jump up to: 6.0 6.1 6.2 6.3 6.4 Inderjit Kaur, Prempal Kaur, Abhishek Handa, Priya Agrawal, “Diagnostic and Therapeutic Role of B Scan Ultrasonography in Traumatized Eyes”. Journal of Evolution of Medical and Dental Sciences 2014; 3:3543-50
- ↑ Puodžiuvien E, Paunksnis A, Kurapkien S, Imbrasie D. Ultrasound value in diagnosis, management and prognosis of severe eye injuries 2005; 3:56.
- ↑ Pavlin CJ, Harasiewicz K, Sherar MD, Foster FS. Clinical use of ultrasound biomicroscopy. Ophthalmology 1991; 98:287–95
- ↑ Nouby–Mahmoud G, Silverman RH, Coleman DJ. Using high-frequency ultrasound to characterize intraocular foreign bodies. Ophthalmic Surg 1993; 24:94 –9
- ↑ Vincent A. Deramo, Gaurav K. Shah, Caroline R. Baumal, Mitchell S. Fineman, Ze´lia M. Correˆa et al. Ultrasound Biomicroscopy as a Tool for Detecting and Localizing Occult Foreign Bodies after Ocular Trauma. Ophthalmology 1999; 106:301–305
- ↑ John A. McWhae, Andrew CS, Crichton and Monique Rinke.Ultrasound biomicroscopy for the assessment of zonules after ocular trauma: Ophthalmology 2003;110:1340–134
- ↑ Huang D, Swanson EA, Lin CP, et al. Optical coherence tomography. Science 1991; 254:1178 –1181
- ↑ Mahendradas P, Vijayan PB, Avadhani K, Garudadri S, Shetty BK. Usefulness of anterior segment optical coherence tomography in the demonstration of intralenticular foreign body in traumatic cataract. Can J Ophthalmol. 2010; 45:413–4.
- ↑ Mateo-Montoya A, Dreifuss S. Anterior segment optical coherence tomography as a diagnostic tool for cyclodialysis clefts [letter]. Arch Ophthalmol. 2009:127;109–11
- ↑ Berline R. Zur. Sogenannten commotio retinae. [So-called commotio retinae]. Klin Monatsbl Augenheilkd. 1873; 1:42–78. German
- ↑ Gill MK, Lou PL. Traumatic macular holes. International ophthalmology clinics. Summer 2002;42(3):97-106
- ↑ Azevedo S, Ferreira N, Meireles A. Management of pediatric traumatic macular holes -case report. Case reports in ophthalmology. May 2013;4(2):20-27
- ↑ Wood CM, Richardson J. Indirect choroidal ruptures: aetiological factors, patterns of ocular damage, and final visual outcome. The British journal of ophthalmology. Apr 1990;74(4):208-211
- ↑ Ament CS, Zacks DN, Lane AM, et al. Predictors of visual outcome and choroidal neovascular membrane formation after traumatic choroidal rupture. Archives of ophthalmology. Jul 2006;124(7):957-966
- ↑ Levin, LA, Seddon JM & Topping, T (1991): Retinal pigment epithelium tears associated with trauma. Am J Ophthalmol 12: 396– 400
- ↑ Jump up to: 21.0 21.1 Hart JCD, Frank HJ. Retinal opacification after blunt nonperforating concussional injuries to the globe; a clinical and retinal fluorescein angiographic study. Trans Ophthalmol Soc UK 1975; 95:94–100.
- ↑ Duke-Elder S, MacFaul PA. Contusion effects on the choroid: contusion effects on the retina. In: Duke-Elder S. Editor. System of ophthalmology. Volume 14. Part 1. London: Henry Kimpton, 1972:150–187.
- ↑ Berlin RZ. Zursogennanten commotio retinae. Klin Monatsbl Augenheilkd 1973; 11:42–78.
- ↑ Yamana T. Retinochoroidal lesions in concussional injuries of the eyes: an experimental study. Acta Soc Ophthalmol Jpn 1981; 90:1049–1066
- ↑ Yannuzzi LA, Slakter JS, Sorenson JA, Guyer DR, Orlock DA. Digital indocyanine green videoangiography and choroidal neovascularization. Retina 1992; 12:191–223.
- ↑ Korte GE, Reppucci V, Henkind P. RPE destruction causes choriocapillary atrophy. Invest Ophthalmol Vis Sci 1984; 25:1135–1145.
- ↑ Delori FC, Dorey CK, Staurenghi G, et al. In vivo fluorescence of the ocular fundus exhibits retinal pigment epithelium lipofuscin characteristics. Invest Ophthalmol Vis Sci 1995; 36:718–29
- ↑ Brunk UT, Wihlmark U, Wrigstad A, et al. Accumulation of lipofuscin within retinal pigment epithelial cells results in enhanced sensitivity to photo-oxidation. Gerontology 1995;41(Suppl 2):201–12.
- ↑ Jump up to: 29.0 29.1 Mahesh G, Jain A, Bodhankar P, et al. Imaging in posterior segment ocular trauma. Kerala J of Ophthalm. 2019; 31(2): 92-101
- ↑ Lin K, Ngai P, Echegoyen J, et al. Imaging in orbital trauma. Saudi J of Ophthalm. 2012; 26: 427-432
- ↑ Almousa R, Amrith S, Mani AH, Liang S, Sundar G. Radiologic signs of periorbital trauma – the Singapore experience. Orbit 2020 Dec; 29(6): 307-12.
- ↑ Pinto A, Brunese L, Daniele S, Faggian A, Guarnieri G, Muto M, Romano L. Role of computed tomography in the assessment of intraorbital foreign bodies. Semin Ultrasound CT MRI. 2012 Oct; 33(5): 392-5.
- ↑ Jump up to: 33.0 33.1 Mafee MF, Karimi A, Shah JD, Rapoport M, Ansari SA. Anatomy and pathology of the eye: role of MR Imaging and CT. Magn Reson Imaging Clin N Am. 2006; 14(2): 249-70