Facial Reanimation
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
Facial Reanimation
Background
The facial nerve is responsible for movement of the face including vital components of daily activities like smiling, blinking, chewing and eating. Loss of facial nerve function with resultant facial paralysis is debilitating, leading to negative functional and social sequelae. Facial reanimation is a surgical approach to restoring mimetic function to the face.
Impaired eyelid closure from facial paralysis can cause morbidity from functional ophthalmic conditions like lagophthalmos, exposure keratitis, recurrent corneal ulcers, and in severe cases, vision loss. Additionally, nasal valve collapse and obstruction may occur when the weight of midface tissue is combined with dysfunction of the nasalis and dilator naris muscles[1]. Paralysis of the lower face leads to the inability to smile and difficulty with speech production and oral phases of chewing and swallowing, including dysphagia.
Socially, the loss of facial expressiveness leads to negative interpersonal interactions and self image. Loss of facial expression negatively impacts emotional wellbeing, leading to feelings of isolation, embarrassment, and an overall decrease in quality of life[2].
Although historically, a large focus of facial reanimation has been on the mouth, restoring function and symmetry to the eyes and nose carry significant importance. A study by Dey et al., tracked the eyes of observers when looking at photos of patients with facial palsy and revealed that a large majority of time was spent on the central triangle of the eyes, nose, and the mouth[3]. This indicates that not only functionally, but also socially, there is an overwhelming importance in the reanimation of these specific components affected by facial palsy.
There are multiple approaches to restoring facial symmetry and movement. There are static procedures centered around restoring symmetry and tone, but do not intend to provide movement. However, facial reinnervation and reanimation surgeries targeted at restoring function or creating functional movement similar to the native muscle have emerged as treatment modalities that have shown promising results.
This article aims to address facial reanimation as it pertains to ophthalmologists, as well as alternative static procedures and medical treatments to restore facial symmetry and resting tone.
Anatomy
The facial nerve is also known as the seventh cranial nerve. The facial nerve carries three main nerve fibers: parasympathetic fibers responsible for lacrimal gland function, sensory fibers providing taste sensation to the anterior two thirds of the tongue, and motor nerve fibers important for facial expression.
Structure
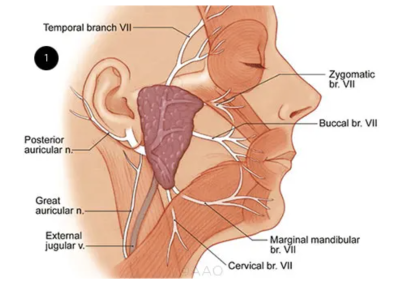
The facial nerve originates from the brain stem. It traverses the facial canal within the temporal bone, emerging through the stylomastoid foramen. Subsequently, within the parotid gland it undergoes division into five terminal branches: temporal, zygomatic, buccal, marginal mandibular, and cervical.
Function
The facial nerve provides motor innervation to the muscles responsible for voluntary facial expressions in addition to the stapedius. Its upper motor neurons are found within the precentral gyrus, and the axons then course through the ipsilateral tract to the lower pons. At this point, the axons intersect to the contralateral side where they synapse on the lower motor neuron [4]. Two essential subnuclei that the lower motor neuron divides into include the dorsal and lateral. The innervations of these two subnuclei are critical in understanding the paralysis distribution in facial nerve palsy. While the dorsal subnucleus innervates the ipsilateral upper facial quadrant, the lateral subnucleus innervates the ipsilateral lower facial quadrant. Moreover, while the dorsal subnucleus is connected to both hemispheres, the lateral subnucleus only receives input from the contralateral corticobulbar fibers. Therefore, when facial palsy impacts the lower motor neuron, the ipsilateral half of the face becomes paralyzed. On the other hand, when facial palsy impacts the upper motor neuron, the contralateral lower quadrant of the face becomes paralyzed. Other muscles that are innervated by the motor nucleus include the auricular, posterior belly of digastric, stapedius, and stylohyoid[4].
The facial nerve contains parasympathetic fibers that supply the submandibular, sublingual, and lacrimal gland secretions. These nuclei are located within the lower pons. The hypothalamus supplies the two parasympathetic nuclei: the superior salivatory nucleus and the lacrimal nucleus.
The facial nerve also contains sensory fibers responsible for the taste sensation from the anterior 2/3rd of the tongue. Additionally, its sensory pathway supplies the external auditory meatus, tympanic membrane and pinna [4]. The geniculate ganglion contains first order sensory neurons of the facial nerve, which become second order in the tractus solitarius within the brainstem. These axons subsequently intersect contralaterally to the thalamus, synapsing on the third order sensory neurons, which finally travel to the postcentral gyrus.
Blood and Lymphatics
The middle cerebral artery supplies the upper motor neuron while the anterior inferior cerebellar artery, a branch off of the basilar artery, supplies the lower motor neuron of the facial nerve. The internal auditory artery supplies the facial nerve once it exits the brainstem. Once the facial nerve transverses the facial canal, it receives its blood supply from the petrosal branch of the middle meningeal artery and the stylomastoid artery[4]. The facial nerve then emerges through the stylomastoid foramen, where the stylomastoid artery becomes the blood supply. Finally, the transverse facial artery artery and the superficial temporal artery supply the facial nerve within the parotid gland[4].
Muscles
The facial nerve innervates the muscles of facial expression, which include the auricularis, corrugator supercilii, depressor anguli oris, depressor labii inferioris, levator labii superioris alaeque nasi, mentalis, nasalis, occipitofrontalis, orbicularis oculi, orbicularis oris, procerus, risorius, zygomaticus major and minor, and others. There are a total of 40 muscles of facial expression[5]. Other muscles innervated by the facial nerve include the stylohyoid muscle, the posterior belly of the digastric muscle, and the stapedius muscle[6]. The stylohyoid is responsible for initiating swallowing and elevating the tongue, the digastric for complex jaw movements, and the stapedius for stabilizing the stapes and regulating sound.
Facial Palsy
The causes of facial palsy are broad and varied. While onset can occur within hours to days, all degrees of facial palsy are undesirable for the patient and can cause a significant amount of morbidity.
Epidemiology
The annual incidence of Bell’s palsy is reported to be between 18 and 40 cases per 100,000 people[7][8]. In children, the incidence of facial nerve disorders is 2.7 per 100,000 people for children under 10 years of age and 10.1 per 100,000 people for people until the age of 20[9].
Larger studies investigating the incidence have reaffirmed the incidence at around 29 per 100,000 people and demonstrate the most affected age group are those between 30 to 45 years old[7][8][10]. There is no specific gender predilection; however, there is an increase in incidence in the winter months[8].
Differential Diagnosis
The most common cause of facial palsy is viral-associated facial palsy, which accounts for about 40-75% of cases. Often presenting with hemifacial weakness, facial palsy that has an acute onset of less than 72 hours is generally termed Bell’s Palsy after Scottish Neurologist Charles Bell. Bell’s palsy may present with a prodromal phase and evolve over 1 to 3 days after. It typically presents unilaterally with 70-90% of patients achieving spontaneous recovery[7].
Other etiologies and considerations that should be on the differential include infectious causes of facial palsy like Ramsay Hunt Syndrome from Varicella Zoster Virus, lyme disease from Borrelia Burgorferi, and HIV-associated facial palsy.
Longstanding facial palsy should raise suspicion for congenital causes such as birth trauma, syringobulbia causing mass effect, Mobius syndrome, Goldenhar-Gorlin Syndrome, geniculate ganglion vascular malformations, and pontine vascular malformations[8].
Otologic causes relate to acute otitis media, malignant otitis externa, and cholesteatoma. Additionally, there are neoplastic etiologies which can cause compression and disruption to normal nerve function including acoustic neuromas/ vestibular schwannomas, hemangiomas, facial nerve schwannomas, and leptomeningeal carcinomatosis.
Facial trauma or iatrogenic injury are common causes of facial palsy in which the facial nerve has been lacerated or damaged. With invasive skin cancers, parotid tumors, or temporal bone lesions, it is of utmost importance to obtain proper margins. Therefore, the facial nerve is often compromised during surgery. Trauma with temporal bone fractures, or other surgeries involving the parotid area or otologic surgeries should always be included in the differential[11].
Similarly, cerebrovascular accidents should be considered when acute facial palsy is identified.
Finally, systemic autoimmune diseases should not be discounted as they also contribute to the causes of facial palsy. The differential should include: sarcoidosis, Melkersson-Rosenthal Syndrome, Guillain-Barre, multiple sclerosis, amyloidosis, granulomatosis with polyangiitis, Sjogren syndrome, Systemic Lupus Erythematosus, and Bechet disease[8].
Physical Examination
In addition to a full ocular examination, clinical examination should encompass more than the traditional ophthalmic examination and close attention should be given to the periorbital region, entire face, and neck.
The physical exam should also include the following components[8]:
- Examination of the head and neck, with special attention to the skin
- Skin examination should assess for rashes and vesicles (zoster), nodules, and lesions (skin cancer, systemic, autoimmune, lyme disease)
- Cranial Nerve Examination
- With special attention to the asymmetry between CN 7. The face should be separated and evaluated in thirds to note symmetry of the forehead, midface, and lower face
- Attention to the brow height and symmetry between the sides
- Attention to eye closure, vertical eyelid height, lagophthalmos, corneal reflex, and bell phenomenon
- The lower eyelid skin should be examined closely, with particular attention to elasticity with a snapback test to illustrate orbicularis oculi tone
- Fluorescein staining of the eye and Schirmer test to evaluate for sufficient corneal protection
- Palpation of the parotid region to evaluate for parotid masses
- Examination of the tongue for fissures assessing for Melkersson-Rosenthal Syndrome which may have recurrent facial palsy
- Examination of the mid cheek and fat pad for flaccid ptosis
- The nasolabial fold should be assessed as well as oral commissure for inferior displacement
- Facial movement should be assessed for the level of flaccidity- ranging from completely flaccid to partial, and partial with synkinesis
Diagnostic Tests/ Imaging
Obtaining the proper diagnosis is paramount to planning medical or surgical treatment. Thorough history taking is important and special attention should be given to time of onset, family history, and recurrence.
Laboratory Workup
Given the many potential causes of facial palsy, laboratory workup is often necessary depending on the highest degree of suspicion on the differential diagnosis. As listed above, there are many autoimmune and infectious etiologies where laboratory testing may aid in diagnosis.
Laboratory tests that may aid the physical examination are listed below[7]:
- CBC
- ESR
- CSF analysis
- Glucose
- TSH
- CRP/ESR
- ANCA
- ACE
- RF
- ANA
- SSA/SSB
- FTA-ABS/RPR/VDRL
- HIV
- Antiphospholipid Ab
Imaging
Much like the laboratory workup, imaging plays an important role in evaluating the facial nerve. CT and MRI can serve a role in identifying many of the causes of facial palsy. CT is especially useful for bony abnormalities and architecture of the temporal bone. CT is helpful to image the lateral course of the facial nerve from the porus acusticus to the stylomastoid foramen [12].
MRI is particularly helpful for excluding particular pathologies and for soft tissue or inflammatory abnormality identification. MRI is also more advantageous to image the facial nerve from the brainstem to the fundus of the internal auditory canal and to further characterize parotid malignancy. Normally, the facial nerve in the cisternal, intracanalicular, labyrinthine, and parotid segments does not enhance; therefore, any abnormal enhancement should raise suspicion for inflammatory or malignant etiologies [12].
The list below can show specific T2 hyperintensities that may provide further evidence to aid in diagnosis[7]:
- Encephalopathy
- Transient Ischemic Attack
- Sarcoidosis
- Small Vessel Vasculitis
- Behcet Disease
- Infection (fungal, parasitic, lyme, viral)
- Neoplasm (gliomas, lymphomas)
- Trauma
Electrophysical testing
There is a role for facial nerve testing to assess the degree of damage and the viability of the facial nerve, muscle fibers, and motor units. The outcomes of these tests will aid the surgeon in selecting the appropriate surgical approach. The most common tests used are the Nerve excitability test (NET), Maximal stimulation test (MST), Electroneurography (ENoG), and Electromyography (EMG).
Described by Laumans and Jonkees in 1963, the nerve excitability (NET) test has been well-studied and applied in the clinical setting [13]. This test is performed by discovering the lowest current needed to elicit a facial twitch. This level is set as the threshold of excitation. The difference in thresholds is then compared between the contralateral sides of the face.
ENoG functions by analyzing the compound muscle action potential (CMAP) of the facial muscle after it has been stimulated. The trunk is stimulated maximally with a bipolar stimulator and the action potential is recorded. Facial nerve testing with ENog is typically performed in the nasolabial fold, because it is the most reliable site. ENog Should ideally be performed within 72 hours to 21 days after onset of facial palsy. It should also be performed in conjunction with EMG to provide more context. It is also important to note that ENoG is unable to differentiate between neurapraxia and more severe lesions[14].
EMG Can be separated into needle EMG and surface EMG. Needle EMG functions by analyzing a facial MUAP from a needle that is inserted into the facial muscle. The surface EMG differs by performing the recording on the surface of the skin and combines the sum of MUAP within the area of the electrode. EMG differs from ENog time-wise because it provides its maximal utility two to three weeks after the onset of palsy. EMG is advantageous because it can be used to track nerve regeneration after a reinnervation procedure. EMG is the only electrophysiological test which provides diagnostic utility after the nerve has degenerated. NET, MST, and ENoG no longer provide diagnostic utility after approximately two to three weeks of onset of facial paralysis[13][14].
Indications
Indications for facial reanimation are present in cases of facial palsy where there is no chance for spontaneous recovery. Indications also depend on the level of severity of facial palsy and extent of atrophy. If there is a chance for spontaneous recovery after insult, procedures centered around reanimation should be delayed. However, if the mechanism of injury is such that intervention would deem the case “reversible”, then procedures like neurorrhaphy that focus on reinnervation should be performed within a few weeks to months[15].
There is an indication for nerve transfers if there are intact distal facial nerve branches and viable facial muscles. These procedures can be performed within 2 years of initial paralysis, but attempting the procedure after gives low chances that the recipient site will accept the nerve transfer[16].
If there is residual movement in the face, static procedures which offer no restoration of movement may be indicated to prevent negative sequelae and restore symmetry. Lid laxity, lagophthalmos, corneal exposure, and facial asymmetry may be addressed with procedures like upper eyelid loading, lateral tarsorrhaphies, lower eyelid slings, and facial slings.
If there has been significant atrophy and there is poor prognosis for reinnervation, there is an indication for facial reanimation with muscle transpositions, free flaps and muscle grafts. The patient’s age, goals, and expectations should always be reviewed before proceeding.
Preoperative Evaluation
The first step of the preoperative evaluation should focus on categorizing the degree of reversibility as well as the time since onset of facial paralysis. Once facial movement is diminished in the face, there is a time component that is essential to determining the success of reinnervation as well as the modality of treatment for reanimation.
Reversible/ Short-Term Reanimation
In cases where there is recent paralysis to the facial muscles or where the mechanism of injury has not caused irreversible loss of function, the viability of the facial nerve, muscle fibers and motor units must be assessed. This may be accomplished with electroneurography (ENog) or Electromyography (EMG)[15]. In these short term/reversible cases, the goal of therapy is to re-establish and strengthen the neural connections to the muscles[17]. The approach therefore is targeted toward utilizing functional motor units with nerve grafts, whereas there would be low utility and response in such an approach for atrophic muscle.
Irreversible/ Long-Term Reanimation
When there has been an irreversible mechanism of nerve injury or longstanding atrophy of musculature, the muscle may no longer be capable of producing fibrillations. In these cases, supplying a new neural stimulus is the incorrect approach and is insufficient to remediate the underlying issue. Once the damage and time has been categorized for these cases, reinstituting a degree of facial symmetry may be approached with static procedures, muscle transpositions, or free muscle transfers[15].
Case for Pediatric Patients
Special consideration should be given during the evaluation due to unique conditions surrounding the pediatric population. There must always be parental input and consent obtained, and foresight should be given to growth and the way the anatomy of the pediatric patient may change over time. Typically, facial surgical procedures are deferred until 5 or 6 years of age and the emphasis is on more conservative methods of eye protection. The literature does not describe any detrimental effects of facial nerve surgeries on facial growth[18].
Facial Nerve Grading Scale
Many different grading scales exist to assess facial nerve function. The lack of a universally adopted system poses challenges between providers and contributes to a source of error for accurate grading. However, the Facial Nerve Disorders Committee of the American Academy of Otolaryngology introduced the House and Brackmann Grading Scale (HBGS) in 1983, and it has remained the gold standard. Other notable scoring scales that exist include the Sunnybrook facial grading system, the Sydney Facial Grading System, the Burres-Fisch System, and the Nottingham System[16].
The House and Brackmann Grading Scale assigns a grade of 1 to 6 in categories depending on their degree of dysfunction. It is associated with 93% reliability, but also has its shortcomings. It does not consider different levels of function of different parts of the face and also fails to account for the level of difference in inter-rater reliability.
The House and Brackmann Grading Scale [19].
| Grade | Description | Characteristics |
| I | Normal | Normal facial function in all areas |
| II | Mild Dysfunction | Gross: Slight weakness noticeable on close inspection; may have very slight synkinesis
At rest: normal symmetry and tone Motion: Forehead - moderate-to-good function Eye: complete closure with minimum effort Mouth - slight asymmetry |
| III | Moderate Dysfunction | Gross: obvious but not disfiguring difference between two sides; noticeable but not severe synkinesis, contracture, and/or hemifacial spasm
At rest: normal symmetry and tone Motion: Forehead - slight to moderate movement Eye -Complete closure with effort Mouth - slightly weak with maximum effort |
| IV | Moderately Severe Dysfunction | Gross: obvious weakness and/or disfiguring asymmetry
At rest: normal symmetry and tone Motion: Forehead - none Eye - incomplete closure Mouth - asymmetric with maximum effort |
| V | Severe Dysfunction | Gross: only barely perceptible motion
At rest: asymmetry Motion: Forehead - none Eye - incomplete closure Mouth - slight movement |
| VI | Total Paralysis | No Movement |
Surgical Approach Preparation
After appropriate imaging, nerve testing, and facial nerve grading has been performed, the surgical approach should be determined. For reinnervation, the surgeon must decide between a neurorrhaphy or a nerve graft depending on the situation.
Neurorrhaphy
Proper technique selection is paramount to ensure the most optimal outcomes. The highest chance for success of recovery is via a neurorrhaphy with primary end-to-end anastomosis of the facial nerve stumps as soon after the time of injury as possible. The surgery to reapproximate the epineurium should be performed ideally within 3 days of insult. Doing so attempts to optimize the number of axons that regenerate while reducing the chance of synkinesis[20].
Physical characteristics of each component of the procedure should be considered to maximize the chances of a successful outcome. The first considerations should include physical length of the neurons intended for neurorrhaphy as well as appropriate access to the surgical site[11].
The preoperative consideration should also account for the possibility of residual tension after neurorrhaphy, which may cause the nerve graft to fail.
Other considerations can be given to adjunct intraoperative steps in the neurorrhaphy. Techniques aimed at improving neuronal regeneration include epineural/perinerual suturing, tissue adhesives, tubulization, and laser neurorrhaphy. However, none of these adjunctive steps have proven to improve outcomes [11].
Donor Site Nerves
Facial Nerve Cross-Face Graft
One option for reinnervating the facial nerve on one side is to use the contralateral facial nerve as a donor. This is achieved by using an interpositional graft, usually the sural nerve due to its length, to connect the two contralateral sides of the facial nerve. One unique advantage of this technique is that the facial nerve from one side may have multiple anastamoses from the segmental branches to allow re-innervation of multiple portions of the face. A popular selection for the donor segment of the facial nerve is the buccal branch. One potential drawback of this technique is that the donor side of the face may experience weakening[15][11].
Hypoglossal Nerve
The hypoglossal nerve can be utilized to provide neural stimulus to the facial nerve. A major benefit of this selection is that it allows for significant motor function when activated, but it requires training on the part of the patient to allow for natural appearing facial emotion[15]. With results appearing as early as 6 months postoperatively, this type of nerve graft also has success rates reported as high as 42-95% [15][21].
However, selecting the hypoglossal nerve also has its drawbacks. The main disadvantages include synkinesis as well as atrophy of the ipsilateral tongue. Another drawback to using the hypoglossal nerve is sacrifice of an intact cranial nerve. The resultant atrophy and disability of the tongue can lead to notable morbidity in the forms of difficulty with mastication, speech and swallowing [11].
Due to the drawbacks found with earlier cases using the hypoglossal nerve, variations of the technique have evolved with time to circumvent some of the main complications. One variation of the technique is to use a partial hypoglossal to facial nerve nerve graft. This is achieved by using the sural nerve as an interpositional graft to join the distal end of the facial nerve to the hypoglossal nerve. This prevents the need to sacrifice an intact cranial nerve. This variation provides around 41% of patients with facial movement, and has resulted in fewer complications. However, patients may experience a longer recovery period, expecting 9-12 months of no function[21][22].
Masseteric Nerve
First described in 2004 by Bermudez and Nieto, the masseteric branch of the trigeminal nerve (CN V3) has been a popular selection for facial reanimation procedures[23]. As more facial reinnervation surgeries have been completed with the masseteric nerve, more data has been released about its success. Earlier data for outcomes of the surgery aligned with the initial study, with complete return of function by 5.6 to 6 months, but in later studies by Wang et al., facial movement returned at around 87 days postoperatively with a grade of 4 or 5 on the Terzis Smile Functional Evaluation Scale[15][24].
The benefit of this technique is the ease in accessing the nerve, the proximity given its anatomic location, and low morbidity at the donor site. The drawbacks include decreased strength with mastication as well as no longer being able to use the gracilis muscle for a free muscle transfer in the future[15].
Interpositional or Cable Nerve Grafts
Interpositional or Cable Nerve Grafts are necessary considerations when restoring nerve function to a distant site from a donor nerve. There should be two selections when preparing for this surgery: selection of a donor site nerve to provide innervation and selection of the donor nerve to be used as an interpositional graft.
There are many potential donor nerve sites that have been used historically to provide reinnervation to the facial nerve including the hypoglossal nerve (CN XII), the ipsilateral facial nerve (CN VII), the contralateral facial nerve (CN VII), and the masseteric branch of the trigeminal nerve (CN V)[25].
There are also certain nerves popular for their selection as interpositional grafts. Their popularity stems from the favorable characteristics including ease of access, length of the nerve available, and axon content[11]. Common interpositional graft nerves include the greater auricular nerve, the sural nerve, and the median antebrachial cutaneous nerve.
There are advantages and disadvantages to the approach. Some favorable advantages include restoring resting muscle tone, allowing for similar mimetic function, and permitting spontaneous facial expression[15]. Restoring innervation with interpositional nerve grafts also provide similar outcomes to direct repairs of the nerves[11].
Disadvantages to the procedure should be considered during surgical preparation, including the challenges of having two anastomosis sites and thus, a longer healing time.
Interpositional Donor Nerves
Greater Auricular Nerve
The greater auricular nerve is a popular selection as an interpositional graft nerve in many reinnervation surgeries, including corneal neurotization. A large reason lies in its ease of access in the neck, its proximity to the face, and its ability to donate around 7 to 10 cm of nerve length[11].
Sural Nerve
The sural nerve is another ideal choice for an interpositional graft. It is largely popular in cases that have longer nerve length requirements, because up to 25-35 cm of nerve can be harvested for the graft. This nerve can be accessed in the lateral leg, 1-2 cm lateral to the saphenous vein. Due to its anatomic distance from the face and neck, it can provide a viable nerve graft if there has been damage close to the face or if the greater auricular nerve has been compromised.
Median Antebrachial Cutaneous Nerve
One advantage of the median antebrachial cutaneous nerve is that it can also transmit sensory innervation[21].
Muscle Transposition
Muscle transpositions are useful techniques that use another functioning muscle to restore mimetic facial movement. It is an effective and reliable technique, but has its strengths and drawbacks. Muscle transpositions are effective and reliable with satisfactory results[16]. One drawback is that there are no naturally occurring movements to the face and the patient needs to undergo training to coordinate facial movements.
The temporalis muscle is often selected for this procedure and modifications can be made with grafts to extend the muscle action to provide movement to the face. Balaji et al, used a modified temporal muscle with a fascia lata graft to pass fibers to the orbicularis oculi and orbicularis oris[26].
Free Muscle Transfers
Free muscle transfers are more invasive, but provide powerful movement to the paralyzed face. Free flaps have a longer operative time and the final outcome of the surgery may take years to manifest. However, it is the gold standard for facial palsy that is longstanding and in the middle third of the face [16]. Some popular muscle selections for this reanimation procedure are the gracilis muscle, the extensor brevis, the serratus anterior, the latissimus dorsi[16].
Surgical Techniques
There are many different variables and potential approaches for facial reanimation. Because each individual case presents unique conditions, the combination of direct and interpositional nerve grafts as well as the many different potential donor nerves and muscle transpositions are vast. The surgical techniques below are not exhaustive for facial reanimation. Rather, they serve to provide examples of commonly performed facial reanimation techniques and major principles that are relevant to the ophthalmologist.
Neurorrhaphy
Many similar principles applied in the neurorrhaphies below overlap in surgical techniques for Corneal Neurotization
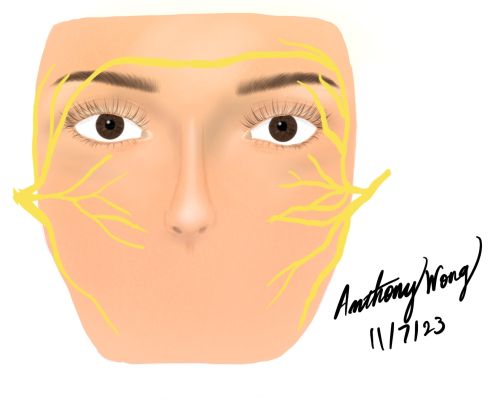
Cross Face Nerve Graft for Reinnervation of the Orbicularis Oculi
When there is injury to the facial nerve on one side of the face, the contralateral side can be used as a donor for neural input. This is advantageous because there are many segments that can be selected as donors. Cross face nerve grafting can also be performed in conjunction with other facial reanimation procedures and the neural input can be used to innervate a free muscle transfer.
Surgical Approach (Adapted from Biglioli et al., [27])
- Prepare and drape the patient in a standard sterile fashion, Note: there will be two: one for the lower extremity and one for the face
- Harvest the sural nerve in the leg, ensuring sufficient length of the graft (approximately 20-25 cm)
- Create a face-lift type incision about 6-8 cm into the temporalis region, extending posteriorly to the earlobe
- Dissect in the subcutaneous plane up to 2cm medial to the anterior margin of the parotid gland
- Open the superficial muscular aponeurotic system (SMAS) a few millimeters ahead of the anterior margin of the parotid gland and under the zygomatic arch
- Continue dissection in an inferolateral to supramedial direction to parallel the direction of the facial nerve
- Identify branches of the facial nerve over the masseter and test the nerves for activation of the orbicularis oculi muscle
- Note: if the branch is not sufficiently large or causes simultaneous activation of other facial musculature, exposure of a separate branch of the facial nerve is necessary
- Isolate a suitable facial nerve branch with the appropriate girth (1 mm)
- Position the sural nerve graft so the the distal end meets with the facial nerve graft and the proximal end is at the paralyzed upper eyelid
- Create and epineural end-to-end coaptation using 11-0 prolene suture
- Position the free end of the sural nerve subcutaneously to reach the paralytic upper eyelid
- With 5-0 nonabsorbable suture, secure the nerve to the deep layers via a cutaneous incision in the upper lid sulcus
- Close the skin
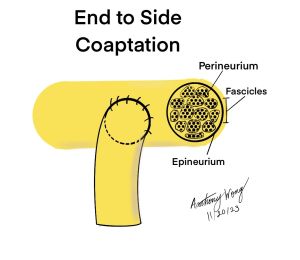
Nerve Transfer With End to Side Coaptation Neurorrhaphy (Hypoglossal-to-Facial Nerve)
Nerve transfers provide neural input from a functional donor nerve to the recipient facial nerve to reestablish movement in the face. These are particularly useful when there is an intact distal facial nerve or facial nerve branches with intact facial musculature. There are many variations with multiple potential donor nerves; however, the hypoglossal nerve is a popular selection and surgical steps are listed below.
Surgical Approach (Adapted from Yoleri et al.,[28])
- Prepare and drape the patient in a standard sterile fashion
- Gain access to the facial nerve via a retroauricular incision
- Note: there are many variations on gaining access to the facial nerve and various segments can be selected based on the approach selected
- Gain access to the hypoglossal nerve via neck dissection below the digastric muscle
- After proper exposure of the hypoglossal nerve, utilize the operating microscope and transect the hypoglossal nerve at a 45 degree oblique angle to expose the proximal axons
- Ensure the main trunk of the distal facial nerve can reach the transected hypoglossal nerve without tension as this will decrease axonal regeneration
- Sever the epineurium and perineurium around the transected area of the hypoglossal nerve to create a perineurial window
- Coapt and attach the large fascicle as well as the satellite fasicles of the facial nerve to the hypoglossal nerve through the perineurial window
- Complete the neurorrhaphy by suturing with 11-0 monofilament nylon and fix the epineurium of the facial nerve to that of the hypoglossal nerve
- Fix the hypoglossal nerve to the surrounding tissues to achieve as minimal tension to the coaptation site as possible
Mixed Neurorrhaphy and Temporalis Muscle Transposition Technique
There exist certain scenarios when combining an acute reanimation technique with a longstanding paralysis reanimation technique can be applied. In cases where there is relatively acute loss of blink with lower loss of function of the face, a masseteric-to-facial-nerve neurorrhaphy plus temporalis muscle transposition may be used. Here, the neurorrhaphy between the masseteric nerve and facial nerve is performed to promptly restore reinnervation to the orbicularis oculi, and the temporalis muscle transposition is aimed at restoring static symmetry and movement of the middle third of the face. This approach is particularly useful for patients who decline to undergo major free flap surgery or where there has been previous surgery or radiation to the face.
Surgical Approach (Adapted from Biglioli et al.[29])
- Prepare and drape the patient in a standard sterile fashion
- Create a face-lift type incision approximately 8 cm into the temporalis region and caudally into the neck crease extending inferiorly to the mandibular border
- Dissect within the subcutaneous plane to 1cm over the nasolabial sulcus
- Place 2-0 nylon stitches and test tension to form an acceptable nasolabial sulcus
- Tease apart the muscles of the masseter muscle anterior to the parotid gland and 1 cm below the zygomatic arch to identify the masseteric nerve approximately 2cm below the surface of the muscle
- Trace the masseteric nerve distally for 2.5-3 cm and cut the nerve
- After gently pulling apart the masseteric nerve, identify the temporalis muscle fibers deep into the masseter
- Osteotomize the coronoid process with piezoelectric surgery to rotate the lower extremity of the temporalis muscle anteriorly and inferiorly by pulling the bone
- Harvest an approximately 6x4 cm fascia lata graft and attach it to the temporalis tendon
- Divide the opposite end of the fascia into strips and attach it to the stitches placed previously
- Note: the fascia lata permits distribution of forces of the temporalis muscle over the entire nasolabial sulcus
- Identify a facial nerve branch that lies anatomically toward the eye and orbicularis oculi muscle
- Sever the facial nerve branch proximally, but ensure there is sufficient length to create a direct neurorrhaphy with the masseteric nerve
- Note: It is very important to avoid creating sharp angles which may inhibit axonal regeneration and growth
- Create an epineural neurorrhaphy with 11-0 suture polypropylene suture
- Fix the curvature of the facial nerve branch with fibrin glue
- Achieve hemostasis and close the skin
Free Muscle Transfer with the Platysma
Multiple procedures exist to restore dynamic movement to the eyes. There have been multiple muscles used as substitutions for the orbicularis oculi muscle. Due to similarity in the musculature, including fiber size, motor unit arrangement, fiber architecture, embryologic origin, the platysma, frontalis, and occipitalis have been proposed to replace the orbicularis oculi muscle. Additionally, the temporalis muscle and the pectoralis minor have been proposed.
Performing a contralateral platysma neurovascular transfer with a cross facial nerve graft has been a popular selection for reanimating the muscles around the eye. The platysma was selected for its adherence to the popular principle of replacing “like with like” due to its similarity to the orbicularis oculi. Variations of this procedure have been attempted, such as by Biglioli et al., who used an avascular piece of the platysma with a cross facial nerve graft.
Surgical Approach (Adapted from Biglioli et al., [27])
Note: this platysma transfer can be coupled as a staged procedure following the cross face nerve graft
- Prepare and drape the patient in a standard sterile fashion
- Create a subcutaneous space within the paralyzed upper eyelid by extending or creating a sulcus incision
- Completely dissect to expose the entire horizontal and vertical height of the eyelid
- Access the previously grafted sural nerve and identify 2-3 cm of nerve graft. Cut the free end with scissors to freshen the nerve
- Create a mid neck skin crease incision on the non-paralytic side and expose the platysma
- Note: avoid coagulation as much as possible to preserve muscle fiber integrity
- Harvest a 5 x 2 cm section of platysma muscle and trim the muscle to obtain a trapezoidal conformation
- Position the muscle so the large base of the trapezoid lies along the upper half of the orbital margin and the small base of the trapezoid lies along the eyelid margin
- Fix the graft in place with 5-0 absorbable monofilament suture
- With the operating microscope, divide the free end of the sural nerve graft into two to four fascicles
- To achieve direct innervation of the muscle, fix the fascicles into the platysma graft with 8-0 stitches
- Close the skin
Static and Alternative Treatments
There are many alternative or adjunct procedures and treatments to facial reanimation that aim to restore facial symmetry and prevent ocular sequelae in the setting of facial palsy.
Facial Nerve Decompression
Facial nerve decompression is a technique utilized for acute facial paralysis due to the compression and inflammation of the facial nerve against bone, that has been active for less than 3 weeks. There are three approaches depending on the localization of the trauma: transmastoid, middle-fossa, or translabyrinthine. Craniotomy is typically done through the transmastoid or middle-fossa approach. If the facial nerve injury is limited to the tympanic or mastoid segments, then the transmastoid approach is done. In this surgical technique, the lateral semicircular canal, fossa incudis, and digastric ridge serve as landmarks for 180 degree decompression of the facial nerve[30]. If the trauma to the facial nerve reaches the labyrinthine segment, the middle fossa approach is then done. The vertical crest, dividing the superior vestibular nerve from the facial nerve, the superior semicircular canal, and the greater superficial petrosal nerve all serve as critical landmarks[30]. In severe cases where the whole intratemporal segment of the facial nerve is impacted, the translabyrinthine may be done. Overall, the middle cranial fossa technique is supported by the greatest amount of evidence indicating effectiveness for Bell’s palsy. Therefore, Bell’s palsy is an indication for the middle fossa approach, where the labyrinthine segment and perigeniculate region are decompressed.
Surgical approach for middle cranial fossa technique (Adapted from Yetiser et al.[31])
- A postauricular incision is made along the mastoid apex, extending upward toward the upper part of the auricle.
- Approximately 1 cm superior to the auricle, the incision is continued anteriorly for ~ 3 cm, and then finally extended posteriorly for ~ 4 cm.
- A temporalis muscle flap incision is made.
- A mastoidectomy followed by a posterior tympanotomy to remove the incus is completed to decompress the facial nerve anterior to the lateral semicircular canal from the second genu to the geniculate ganglion under the malleus.
- A 4x5 cm craniotomy is done 1 cm above the mastoidectomy in order to decompress the facial nerve from geniculate ganglion to the internal auditory canal.
- CSF is released through an incision in the dura over the internal auditory canal.
- The sheath is cut along the nerve after complete decompression, followed by soaking the nerve in steroid gel.
- Fibrin glue is used over the internal auditory canal to secure the muscle.
- The removed incus is placed back into position and fixated with glue between the malleus and head of the stapes.
- The remainder of the wound is closed.
Implantable devices
Implantable devices, using an implantable actuator, present as a novel technique for restoration of blink in those with facial paralysis. Hasmat et al. studied the bionic lid implant for natural closure (BLINC), comparing blink restoration in cadaveric models to those of healthy individuals[32]. This implantable decide essentially applied an eyelid sling to a magnetic actuator to demonstrate the capability to effectively restore physiological blink[32]. In another study, Bobrov et al. utilized an implantable bioelectrical closed-loop system in a rabbit model in order to restore the orbicularis oculi muscle and show efficacious restoration of blink in rabbits with unilateral facial nerve paralysis[33].
Surgical technique (Hasmat et al.[32])
- An eyelid sling is combined with a magnetic actuator in order to comprise the implantable device.
- The implantable device is placed within the temporal fossa and fixed to the zygomatic arch. The magnetic core is secured to the lateral end of the sling.
- The magnetic actuator is energized via either a steady current flow using a DC power supply or high pulse energy using a capacitor.
- The magnetic actuator is charged to 10 V.
- The time to complete closure and degree of closure are measured
Static Facial Sling
An alternative to facial reanimation is a static facial sling procedure to help restore facial symmetry and resolve negative sequelae from facial paralysis. The procedure effectively suspends the face to target facial drooping; however, it lacks the ability to restore dynamic movement in the face. A benefit of this procedure is that it can help target the consequences of paralytic ectropion, lower lid laxity, and exposure keratopathy. Additionally, it suspends the nasolabial fold and the oral commissure up to the zygomatic arch and temporal fascia[34].
There are four popular materials that have been used for this procedure: autologous tissue grafts, synthetic grafts, allografts, and suspension sutures[34].
The autologous grafts are advantageous because there is less risk of foreign material rejection, inflammation, infection and extrusion. A downside to using an autologous graft is the increased healing time, surgical time, and need for a donor site. The selection of which autologous graft material to use has varied between the palmaris longus tendon to the tensor fascia lata (TFL). A popular selection is the tensor fascia lata which has been used successfully to elevate the lower eyelid, nostril, lips, and lateral commissure[34].
Synthetic materials are also potential selections for a facial sling, and a common selection is Gore-Tex (Expanded polytetrafluoroethylene, Gore-Tex, Gore & Associates, Flagstaff, AZ). The advantages lie in its readiness, biocompatibility, and ease of manipulation. Disadvantages lie in the potential for stretching and loss of structural tension over time. Although a correction factor may be planned pre-operatively, the variation between person to person for relaxation is unpredictable.
Allografts have also been used for the static facial sling and potentially avoid the drawbacks faced with other materials. A popular selection is acellular human dermis (AlloDerm, Lifecell Bridgewater, NJ). Acellular dermis has been used for a variety of procedures ranging from nasal septum perforation repairs to duraplasty[35]. Like autologous graft material, allografts have low risk of rejection, infection, and extrusion due to their excellent biocompatibility. Like synthetic materials, there is a concern for stretching after application of tension, but according to the literature it is to a lesser extent than Gore-Tex
Surgical Technique adapted from Mehta et al., [30] Tate et al.,[11] Langille et al., [34])
- Prepare and drape patient in sterile fashion
- Select appropriate graft material (autologous tissue grafts, synthetic grafts, allografts)
- Create an incision to gain access to the region where the lower border of the sling will lie. This may be accomplished with an incision in the vermillion border
- Create a tunnel in the subcutaneous, sub-SMAS, or deep planes between incisions in the zygomatic region and near the oral commissure
- Attach the graft material to the midface soft tissues and superiorly to the zygomatic arch or lateral orbital wall with suture, plates, or screws
- Ensure that the tension is applied with the correct posterior-superior force vector
- Close the incision sites
Suture Suspension Technique (adapted from Alex et al. [36])
- Prepare and drape the patient in sterile fashion
- Gain access to the the lateral orbital wall via incisions in the skin along the and drill holes along the lateral orbital rim
- The holes should allow for a 3-0 gauge long Keith needle to pass
- Using a long Keith needle, pass suture from the lateral canthus under the midface soft tissues to the periorial/perialar area
- To address paralyic ectropion and lower lid laxity from facial paralysis, attach the lateral canthal tendon to the same bone tunnel created previously
- Close the skin
Because the superior point of fixation is the lateral orbital wall/zygomatic region, midface suspension of the soft tissues can often assist with paralytic ectropion or lower eyelid laxity. However, there are more periorbital specific procedures like the lower eyelid sling to address these issues.
Lower Eyelid Sling
A static procedure focused more on the periorbital region is the lower eyelid sling. Sharing many of the same principles as the facial sling, this is more targeted to fixing lower lid malposition and laxity, and can be especially useful if there is concurrent medial and lateral canthal tendon laxity.
Surgical Technique with fascia lata graft (adapted form Morisada et al.,[37] )
- Prepare and drape the patient in a sterile fashion
- Obtain a fascia lata graft from the lateral thigh or palmaris longus tendon. The graft should be cut to approximately 35mm x 10mm.
- To obtain the lateral thigh fascia lata, an incision should be made 6cm above the lateral tibial condyle
- Achieve exposure of the inferior tarsus, medial canthal tendon, and lateral canthal tendon. This can be achieved via a combination of a subciliary incision, lateral canthotomy/ cantholysis, or medial transcutaneous incision.
- Use the fascia lata graft to attach the tarsal plate medially to the nasal bones and supero-laterally to the orbital rim with 5-0 polydioxanone
- Close the skin incisions of the canthotomy/canthoplasty with 5-0 fast gut suture
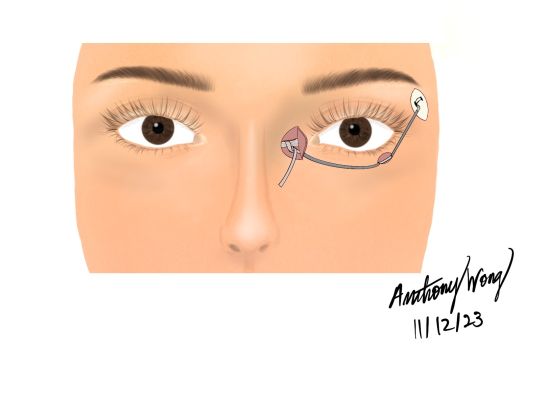
Eyelid Weight Placement
In cases of defective or incomplete eyelid closure, the use of a gold or platinum weight implant can be useful to remedy the deficit. With lagophthalmos, the cornea is continuously exposed, which consequently leads to painful long term complications such as keratitis, conjunctivitis, and ulceration. This technique is meant to prevent this, and ultimately improve blinking, eyelid closure, and protection of cornea. It incorporates the transcutaneous insertion of a gold plate into the upper eyelid, typically into the pretarsal space.
The plate is placed posterior to the orbicularis oculi muscle, which is usually the paralyzed muscle causing the lagophthalmos. Due to the thin nature of the lid, a possible difficulty to this procedure includes the extrusion or noticeability of the implant. Platinum plates have been growing in favor due to superior cosmetic outcomes[30]. Unlike gold, which contains a density of 19.3 g/cm3, platinum has a density of 21.45g/cm3, thus making it a denser and more compact metal at room temperature[38]. In other words, a gold plate requires greater space or volume than platinum, which is difficult as the upper lid is a thin space and will as a result cause a bulky cosmetic outcome[38].
To further improve cosmetic outcomes of the procedure, postseptal implantation is further being studied as a superior method associated with fewer complications and more concealed outcomes[38]. Another possible complication of this procedure includes the unwanted formation of a capsule around the plate, which would require subsequent removal. The benefit is that the procedure is reversible, and If eyelid function returns or adverse reactions occur, the plate may simply be removed.
Surgical approach (Adapted from Morisada et al. ,[37] and Oh et al.,[38]):
- An estimated weight using either Gold or Platinum is first taped to the upper lid, with the patient practicing blinking to determine the correct weight to be surgically placed.
- The preoperative skin incision is marked along the pretarsal skin crease, approximately 7-10mm above the eyelashes, and at the midpupillary line.
- 1% lidocaine with epinephrine is used for local anesthesia using a 30G needle.
- Using a No. 15 blade, the initial cut is made in the supraciliary crease deep to the orbicularis oculi to expose the tarsal plate.
- Metzenbaum scissors are useful for precise dissection to create a compartment for the weight. The levator aponeurosis is dissected and the plate is placed into the space between the levator aponeurosis and the orbital septum.
- A 6-0 nylon or vicryl suture is used to attach the weight to the tarsus, utilizing at least 2 sutures.
- The patient can be moved to a sitting position and instructed to open and close their eyes in order to evaluate proper placement and positioning of the weight.
- The final skin closure is completed using 6-0 silk or prolene sutures.
Palpebral Spring Procedure
The placement of a palpebral spring is a possible alternative, however this procedure is difficult to achieve and still has very limited sources of study done[38]. The mechanism of the spring consists of passive closure of the upper lid, while then permitting the levator muscle to actively overcome the spring and open the lid[39]. While weighted plates utilize gravity, springs restore blink without conscious intention. The spring consists of an elastic stainless steel wire comprising 18% chrome and 8% nickel[39]. The procedure is done under local anesthesia and the patient assists with testing the correct placement of the spring before securing the inferior arm into place to the tarsal plate.
Surgical approach (Adapted from Guy et al.[39])
- Preoperative markings are made in 3 spots: middle third of the superior orbital rim, lateral orbital rim halfway between the lateral canthus and lateral eyebrow, and superior to the lid margin over the tarsal plate
- Local anesthesia is given with epinephrine for bleeding control.
- Incisions are made along the preoperative markings.
- The tarsal plate and periosteum of orbital rims are exposed.
- The superior arm of the spring is adjusted to fit the superior orbital rim, and the inferior arm of the spring is adjusted to fit the tarsal plate.
- The arms of the spring are inserted using a 20 G needle and guided subcutaneously into position.
- Non-absorbable sutures are used to attach the central coil to the periosteum of the lateral orbital rim.
- Non-absorbable sutures are used to secure the superior arm to the periosteum.
- The patient can now be instructed to open and close their eyes in order to access placement and position.
- The spring’s strength can be adjusted by opening or closing the lateral coil, and placement of the spring may be adjusted at this time as well
- Once pleased with the final positioning, strength, and placement of the spring, non-absorbable sutures are used to secure the inferior arm to the tarsal plate.
- An ophthalmic antibiotic ointment is applied prior to adding a sizable dressing, which can be removed 2 days postoperatively.
Lateral Tarsorrhaphy
Lateral tarsorrhaphy is another procedure that can be used in cases of inadequate eyelid closure in order to protect the cornea. There are two forms of this procedure: temporary and permanent. For short-term tarsorrhaphy, two horizontal mattress sutures are used over bolsters to close the eye, which can be opened and closed during eye exams. This technique lasts for approximately two to eight weeks. On the other hand, a long-term tarsorrhaphy involves joining of the lateral third of the upper and lower lid using interrupted sutures to keep central lid opening, permitting the patient to maintain visibility. In this method, 1mm of the posterior lamella is excised, followed by the posterior and then anterior lamella closure.64
Surgical approach for temporary tarsorrhaphy (Adapted from Rajak et al.[40])
- 1% lidocaine with epinephrine is used for local anesthesia and iodine is used to sterilize the field.
- Sutures are tied over bolsters in order to protect the skin from being cut. A total of two 2 cm and one 1 cm bolster is needed.
- One of the 2 cm bolsters is aligned over the upper lid with a suture placed through it and the skin 3-4 mm above the lid margin, through the tarsal plate. The needle is then passed through the lower lid and again into the tarsal plate, exiting the skin 2-3 mm below the lower eyelid margin.
- Another 2 cm bolster is aligned along the lower lid with a 1 cm bolster on top of it. A suture is passed through the upper bolster, then upper lid, followed by lower lid and finally the lower bolster.
- Move the two lower lid bolsters superiorly to shut the lids. The 1 cm bolster is used to secure the lid closed. The 1 cm bolster can be pulled back down in order to open the lids.
Surgical approach for permanent tarsorrhaphy (Adapted from Rajak et al.[40])
- 1% lidocaine with epinephrine is used for local anesthesia and iodine is used to sterilize the field.
- Using a No. 11 or 15 blade, separate the anterior and posterior lamella. Using the blade or scissors, expand the cut for about 5mm.
- 1 mm of the posterior lamella is removed, which removes the epithelium and allows for improved healing.
- The posterior lamella is closed using an absorbable 5-0 or 6-0 suture. The needle is first passed through the posterior lamella of the upper lid, and then through the posterior lamella of the lower lid. This is again with a second suture.
- The anterior lamella is closed using a 4-0 or 6-0 suture. The needle is passed through the upper lid, 2-3 mm above the lid margin, and then into the anterior lamella of the lower lid, exiting 2-3 mm below the lid margin. This step is done at least twice with the sutures set 3 mm apart until the eyelid skin completely closes over the posterior lamella
Upper Eyelid Blepharoplasty
Upper eyelid blepharoplasty is a procedure that can be utilized in patients where muscle paralysis causes ptosis. If there is a lesion that impacts the facial nerve, such as in Bell’s Palsy, leading to a weakened orbicularis oculi muscle, a blepharoplasty can create lid symmetry and reduce visual impairment.
Surgical approach (Adapted from Patel et al.[41])
- Preoperative markings are made along the upper eyelid crease.
- 1% lidocaine with epinephrine is used for local anesthesia. It is important to make sure that the levator muscle is not injected during this. If the patient is pain intolerant, the procedure can be accomplished in the operating room under conscious sedation.
- A 15 Bard-Parker blade is used to make the marked incision along the upper eyelid crease.
- A strip of the orbicularis oculi muscle is removed.
- Fat is removed following the opening of the orbital septum.
- The medial fat pad is debulked, with the amount personalized based on the individual's facial features for a desirable aesthetic outcome.
- 6-0 catgut, nylon or prolene interrupted sutures are used to close the incision.
Browplasty
Upper eyelid blepharoplasty is a procedure that can be utilized in patients where muscle paralysis causes ptosis. If the ptosis stems from a weakened frontalis muscle due to facial nerve damage, this would require a browplasty. Patients with facial nerve palsy have had positive results corresponding to endoscopic brow-lifts[42].
Surgical approach (Adapted from Morisada[37] and Servat et al., [43])
- Preoperative markings are made along the bilateral temporal crescents and orbital rims. Another mark is made 2 cm around the bilateral supraorbital notches to safeguard the supraorbital and supratrochlear nerves. 1 cm behind the hairline a central incision is marked, and 4.5 cm lateral to this central incision the paramedian incisions are marked. Lastyle, bilateral marks are marked in the temporal areas.
- 2% lidocaine with epinephrine or 0.75% bupivacaine with epinephrine is used for supraorbital nerve block. For those who are pain intolerant, general anesthesia can be used to perform the procedure.
- A 15 blade is used to remove the temporal skin, followed by dissection to uncover the deep temporal fascia
- The endoscope is guided through the temporal compartments.
- The lateral canthal ligament is separated.
- The central and paramedian cuts are made all the way to the periosteum, which is then loosened.
- Blunt dissection is used to separate muscle from tissue. The corrugator supercilli, procerus, and depressor supercilli muscles are avulsed. The bilateral temporal crescents are released.
- After sufficient dissection is completed for sufficient periosteum release, 4-0 monocryl sutures are used to reapproximate the subcutaneous tissue. Fixation is done using biodegradable implants to secure the skull.
- 6-0 sutures absorbable gut, prolene, or nylon sutures are used to close the superficial temporal fascia and final skin. Staples can also be used to close the final skin.
Lateral Tarsal Strips
A paralytic lower lid ectropion, causing difficulty blinking and complete eye closure, can be treated with lateral tarsel strips. The ultimate goal of this technique is to shorten and tighten the lower lids in order to restore blink in order to keep the cornea moist and protected. This procedure can be done in-office under local anesthesia. Possible complications of lateral tarsal strip placement include hemorrhage, infection, and wound dehiscence[44].
Surgical approach (Adapted from Morisada et al., [37] and Jue et al.,[44])
- Local anesthesia is used
- A lateral canthotomy is done with a 1 cm extension toward the lateral orbital rim.
- The inferior crus is cut in order to carry out a lateral cantholysis.
- A dissection is done to separate the anterior from the posterior lamella.
- The mucocutaneous junction is superiorly cut to construct a lateral tarsal strip. The epithelium is polished off using a 15 blade
- The tarsal strip is securely stitched to the periosteum of the lateral orbital rim using 5-0 nylon.
- 5-0 monocryl sutures are used to stitch the canthotomy. 6-0 gut sutures are used to reconstruct the lateral canthal angle. 6-0 silk sutures are used to close the final skin.
Modified Tarsoconjunctival Flap
In this technique, an upper lid tarsoconjunctival flap is fitted into the lower lid in order to fix paralytic ectropion. A lateral strip canthoplasty or canthopexy is usually done in conjunction with this method. Unlike a canthoplasty or canthopexy itself, this technique addresses the vertical vector laxity that other surgical methods cannot[45]. Due to a weakened orbicularis oculi muscle, the upper and lower lid retractors are left unopposed leading to lagophthalmos and malposition [37][45]. Essentially, a modified tarsoconjunctival flap works to balance these unopposed retractors. The distribution of the tear film enhances with this method, as the coupling allows for the retractors to raise the lower lid and lower the upper lid. Moreover, unlike a tarsorrhaphy that is the original lid coupling technique, a modified tarsoconjunctival flap causes less obvious deformity and prevents the obscured lateral visual field[37][45].
Surgical approach (Adapted from Morisada et al.,[37] and Dedhia et al.,[45])
- A tarsal strip is created via a canthoplasty or canthopexy, or lateral retinacular suspension is performed in order to tighten the lower lid. A 5-0 polydiaxanone suture is used and the suture suspension of the lateral canthus is left untied until the completion of the tarsoconjunctival flap.
- The tarsoconjunctival flap is elevated from the upper lid with the use of a 4-0 silk traction suture. The lid is inverted using a Desmarres retractor. 1% lidocaine with epinephrine is used for hydrodissection to aid the flap elevation.
- Depending on the degree of lid laxity, the superiorly based flap is purposefully designed to be within the range of 3 to 8 mm wide and 4 mm tall.
- A 15 blade is used to incise the lower lid and create a compartment to put in the tarsoconjunctival flap.
- The transconjunctival flap is elevated and a 4-0 interrupted vicryl suture is used to secure it to the compartment. In order to avoid the complication of ocular irritation, it is important to tie the knots away from the sclera.
- The suspension suture that was left untied from the canthopexy or canthoplasty can now be tied down.
- A 6-0 gut suture is used to recreate the lateral canthal angle.
- A 5-0 monocryl suture is used to approximate the edges.
- A 6-0 silk suture is used to close the final skin layer.
Botulinum Toxin (Botox)
Botulism toxin type A has been a longstanding treatment to improve the asymmetry caused by facial paralysis. Clostridium botulism is the anaerobic bacterium utilized in this injection, and its neurotoxin blocks acetylcholine release thus inhibiting the muscle from contracting and causing its paralysis. The goal of injecting botulinum toxin into the non-paralyzed side of the face is to reduce hyperkinesis and subsequently restore normal facial features[46]. Differing muscle points require varying points of injection, dosages, and techniques that are described below.
Procedure technique (de Sanctis Pecora et al.[46])
- Sterilize the areas of injection with alcohol pads.
- Mark the injection points on the skin.
- If the patient is pain intolerant, numb the skin points of injection using a topical numbing cream.
- Use a 6mm needle to draw up the botulism toxin and use the following dosages and techniques to inject the intended non-paralyzed muscle groups.
- Occipto-frontalis: Draw up 0.5-2 units per point, with a total of 3 to 9 injection points. Angle the needle upward and insert 30% of it. Expand the injection locations outward along the lateral canthus.
- Corrugator supercilii: Draw up 4-6 units per point, with a total of 2 to 3 injection points. Insert the entirety of the needle at the muscle origin, and only 30% of the needle at the muscle insertion.
- Procerus: Draw up 4-8 units, with a total of 1 to 2 injection points. Direct the needle upward and insert half of the needle.
- Depressor supercilii: Draw up 1 to 2 units, with a total of 1 injection point. Direct the needle medially and upward and insert subdermally.
- Orbicularis oculi: Draw up 4 units per point for the lateral radial lines or 2 units per point for inferior lines and lateral end of the brow, with a total of 3 to 6 injections points. Superficially insert 30% of the needle.
- Pre-tarsal: Draw up 0.5 units to 1 units per point, with a total of 1 to 5 injection poinst. Direct the needle intradermally 3 mm below the ciliary margin.
- Nasalis: Draw up 2-4 units per point, with a total of 1 to 2 injection points. Direct the needle at a 45 degree angle intramuscularly within the lateral nasal wall superior third.
- Levator labii superioris alaeque nasi: Draw up 2 units, with a total of 1 injection point. Direct the needle upward while inserting half of it into the naso-facial groove.
- Levator labii superioris: Draw up 1-2 units, with a total of 1 injection point. Direct the need upward while inserting half of it perpendicularly and lateral to the nasal prominence.
- Zygomaticus major: Draw up 1-2 units per point, with a total of 1-2 injection points. Direct the needle superficially while inserting half of the needle perpendicularly. The first injection point should be approximately 2 cm from the oral commissure diagonally to the malar prominence, and the second injection point should be directly on the malar prominence.
- Zygomaticus minor: Draw up 2 units, with a total of 1 injection point. Direct the needle superficially while inserting half of the needle perpendicularly approximately 1 cm superior to the zygomaticus major.
- Orbicularis oris: Draw up 1 unit per point, with a total of 2-6 injection points. Direct the needle superficially approximately 2 mm above the vermilion border.
- Risorius: Draw up 1-2 units per point, with a total of 1 injection point. Direct the entirety of the needle deep and perpendicularly approximately 2 cm medially to the masseter.
- Mentalis: Draw up 2-4 units per point, with a total of 1-2 injection points. Direct the entirety of the needle deep and perpendicularly on the chin prominence, approximately 2 cm from the labium inferius oris.
- Depressor labii inferioris: Draw up 1-3 units per point, with a total of 1-2 injection points. Insert 30-50% of the needle superficially 1-1.5cm from the labium inferius oris and 1 cm from the center.
- Depressor anguli oris: Draw up 2-4 units, with a total of 1 injection point. Direct the half of the needle superficially and perpendicularly superior to the mandibular angle and approximately 1 cm lateral to the oral commissure.
- Platysma: Draw up 2 units per point, with a total of 6-10 injection points. Insert the needle superficially, keeping the injection points about 1.5-2 cm apart across the mandibular margin and platysma bands.
Postoperative Care
Postoperative care for neurorrhaphy or free muscle transfers should focus on rehabilitation and protecting the site of surgery. The patient should be instructed to care for the wound with appropriate dressing changes and follow up appointments as scheduled.
Both an intraoperative and postoperative care consideration, care should be given to minimize tension on the site of neurorrhaphy. Doing so serves to prevent scar formation and promotes regeneration of as many axons as possible[15][11]. This can be achieved by proper preoperative and intraoperative planning. Additionally, in the postoperative period, the patient should be instructed to restrict head movements toward the opposite side of surgery for two weeks after surgery [28].
Patients should also be encouraged to perform exercises centered around gaining control of the mimetic muscles. Referrals to physical medicine and rehabilitation can be sent to assist with the retraining of the newly functional facial muscles.
Postoperative Complications
Postoperative complications are a possibility with each procedure listed in this article. For neurorrhaphy, there is always the possibility that no nerve function is restored. There is a higher risk of nerve graft failure if there are multiple neurorrhaphy sites, and perineural fibrosis is a common finding at the neurorrhaphy junction.
A common issue with using nerve to nerve anastomosis is sacrifice of the donor nerve. With facial nerve to facial nerve or hypoglossal to facial nerve surgeries, there is compromise of a previously functional cranial nerve which results in the creation of a new set of issues. A study by Hammerschlag found that using the hypoglossal nerve to innervate the facial nerve results in 45% of patients with both speech and swallowing deficits[47]. It is one of the sites with high risk for morbidity given the high axon count. There are ways to decrease the chance for these deleterious effects with an end to side coaptation or an interpositional graft, but doing so also reduces the neural input directed toward the reinnervation [48]. Patients who receive innervation from the masseteric nerve generally do not face major loss of function sequelae other than facial volume loss[48]. Although there is always the potential for contralateral facial weakness in a cross face nerve graft, it is not commonly seen in practice.
It is important to note that synkinesis is not an absolute complication, but rather a suboptimal outcome. This can occur if the facial nerve is reinnervated at the main trunk or at a location that is proximal to the pes anserinus[48]. Synkinesis may generate its own set of issues by creating unintentional movement and although there is restoration of movement, there may be an accompanying decrease in quality of life. The pathogenesis of synkinesis is thought to be due to misdirection of axons that begin to innervate different neuromuscular junctions after the insult[48]. In a similar manner, there is always the possibility that the incorrect nerve branch is reinnervated by mistake.
Outcome and Prognosis
Fortunately, 70% of patients with idiopathic facial palsy (Bell’s Palsy) will spontaneously recover. The remaining 30% of patients will vary in terms of recovery and may face varying degrees of facial movement restoration and synkinesis. Additionally, patients with Herpes Zoster-related facial palsy are at higher risk of residual weakness, synkinesis, and longer recovery times[8].
The success rates of facial nerve repair varies among the literature with sources ranging from 5% to 86% [49][50]. There is a large difference in success given the large variability in time to repair as well as surgical approach used for reanimation.
Direct nerve repair with tension-free coaptations within 24 hours of injury have the best results with some reports as high as 84.6%[40]. These patients reported by Malike et al., received end-to-end anastomoses and achieved a grade of </= III on the House-Brackmann scale[40].
Malik et al., performed a study comparing outcomes of primary neurorrhaphy versus interpositional grafts vs facial to hypoglossal nerve transpositions. The study revealed that end-to end primary neurorrhaphy provided the best facial reanimation, followed by interpositional nerve grafts, followed by facial nerve to hypoglossal nerve transposition as graded by the House Brackman scale[51].
Rashid et al, performed direct nerve repair with interpositional grafts among a sample size of 22 patients showed the following results [52]:
- Grade V- 4 patients
- Grade IV- 7 patients
- Grade III - 8 patients
- Grade II- 3 patients
Additionally Mohamed et al., found the following results for patients with interpositional grafts in 11 patients after a 2 year follow up period [53]:
- Grade IV- 3 patients
- Grade III- 8 patients
Expected recovery after neurorrhaphy can range from 4 to 9 months, although patients can continue to regain function up to years later[20]. For interpositional nerve grafts, axonal regeneration can be expected at a rate of ~1mm/day. However, this is variable depending on the technique used. With regard to function, facial tone is expected after 6 months and facial movement can be expected at around 7 to 9 months[54].
Studies using a cross facial nerve graft with a masseteric coaptation revealed nerve activation after approximately 2-4 months. The cosmetic results of the study were overwhelmingly positive with one patient having moderate results, five patients having good results and two patients having excellent results [55].
For hypoglossal nerve cable grafts, literature reports list success rates from 42% to 95% with the appearance of volitional motion and resting tone as early as 6 months [15]19. The partial hypoglossal nerve transfer, using an interpositional sural nerve graft to connect the facial nerve with the hypoglossal nerve has success rates of around 41% of patients with good facial movement. There have been fewer complications ; however, there may be a longer recovery period, with 9-12 months without mimetic function[21][22].
Data of early surgeries using the masseteric nerve for facial reanimation showed complete return of function by 5.6 to 6 months. More recent studies by Wang et al. illustrated that movement returned at around 87 days postoperatively with a grade of 4 or 5 on the Terzis Smile Functional Evaluation Scale[15][24].
Outcomes of dynamic vs static procedures
Studies directly comparing orbicularis oculi reanimation with masseter-to-facial nerve transfers compared to static interventions have shown a statistically significant difference in conferring corneal protection.
Remarkably, patients who undergo orbicularis oculi muscle reanimation with masseter-to-facial nerve transfers are on average more able to achieve full eyelid closure compared to patients who undergo traditional static procedures. In a study comparing these two groups, at 21 months, 89% who received orbicularis oculi reinnervation were able to completely close the eyelid as compared to 7.1% of patients who received static procedure treatment[20].
Patients who undergo a combination of static and orbicularis oculi reinnervation tend to have an improved eyelid aperture closure of about 25.3% compared to those who undergo static procedures alone at 9-15 months after surgery[20].
Not only does this surgery improve the overall movement of the eyelid, but the movement dynamics are heavily influenced as well. The same study revealed a 32.8% improvement in closure velocity compared to the static intervention group as well as a 5.6 fold improvement over the initial baseline measurement. Since this surgery remarkably improves eyelid closure, there was a significant advantage in corneal protection versus traditional therapies with 69.9% less corneal damage in mean PEE scores at 18 months. Additionally, compared to the baseline corneal PEE scores, there was 67% less damage. [20]
However, it is important to note that 33% of patients who underwent orbicularis oculi reanimation did develop synkinesis, which necessitated treatment with botulinum toxin to mitigate undesirable effects[20].
References
Add text here
- ↑ Kochhar A, Albathi M, Sharon JD, Ishii LE, Byrne P, Boahene KD. Transposition of the Intratemporal Facial to Hypoglossal Nerve for Reanimation of the Paralyzed Face: The VII to XII TranspositionTechnique. JAMA Facial Plast Surg. 2016 Sep 1;18(5):370-8. doi: 10.1001/jamafacial.2016.0514. PMID: 27348018.
- ↑ Coulson SE, O'dwyer NJ, Adams RD, Croxson GR. Expression of emotion and quality of life after facial nerve paralysis. Otol Neurotol. 2004 Nov;25(6):1014-9. doi: 10.1097/00129492-200411000-00026. PMID: 15547436.
- ↑ Dey JK, Ishii LE, Byrne PJ, Boahene KD, Ishii M. Seeing is believing: objectively evaluating the impact of facial reanimation surgery on social perception. Laryngoscope. 2014 Nov;124(11):2489-97. doi: 10.1002/lary.24801. Epub 2014 Jun 26. PMID: 24966145.
- ↑ Jump up to: 4.0 4.1 4.2 4.3 4.4 Seneviratne SO, Patel BC. Facial Nerve Anatomy and Clinical Applications. 2023 May 23. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan–. PMID: 32119456.
- ↑ Vigliante, C. E. (2005). Anatomy and functions of the muscles of facial expression. Oral and Maxillofacial Surgery Clinics of North America, 17(1), 1–15. https://doi.org/10.1016/j.coms.2004.09.005
- ↑ Dulak D, Naqvi IA. Neuroanatomy, Cranial Nerve 7 (Facial). 2023 Jul 24. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan–. PMID: 30252375.
- ↑ Jump up to: 7.0 7.1 7.2 7.3 7.4 Hohman MH, Hadlock TA. Etiology, diagnosis, and management of facial palsy: 2000 patients at a facial nerve center. Laryngoscope. 2014 Jul;124(7):E283-93. doi: 10.1002/lary.24542. Epub 2014 Jan 15. PMID: 24431233.
- ↑ Jump up to: 8.0 8.1 8.2 8.3 8.4 8.5 8.6 O TM. Medical Management of Acute Facial Paralysis. Otolaryngol Clin North Am. 2018 Dec;51(6):1051-1075. doi: 10.1016/j.otc.2018.07.004. Epub 2018 Oct 5. PMID: 30297178.
- ↑ Ishii LE. Facial Nerve Rehabilitation. Facial Plast Surg Clin North Am. 2016 Nov;24(4):573-575. doi: 10.1016/j.fsc.2016.06.010. PMID: 27712822.
- ↑ Peitersen E. Bell's palsy: the spontaneous course of 2,500 peripheral facial nerve palsies of different etiologies. Acta Otolaryngol Suppl. 2002;(549):4-30. PMID: 12482166.
- ↑ Jump up to: 11.0 11.1 11.2 11.3 11.4 11.5 11.6 11.7 11.8 11.9 Tate JR, Tollefson TT. Advances in facial reanimation. Curr Opin Otolaryngol Head Neck Surg. 2006 Aug;14(4):242-8. doi: 10.1097/01.moo.0000233594.84175.a0. PMID: 16832180.
- ↑ Jump up to: 12.0 12.1 Gupta S, Mends F, Hagiwara M, Fatterpekar G, Roehm PC. Imaging the facial nerve: a contemporary review. Radiol Res Pract. 2013;2013:248039. doi: 10.1155/2013/248039. Epub 2013 May 23. PMID: 23766904; PMCID: PMC3676972.
- ↑ Jump up to: 13.0 13.1 Lee DH. Clinical Efficacy of Electroneurography in Acute Facial Paralysis. J Audiol Otol. 2016 Apr;20(1):8-12. doi: 10.7874/jao.2016.20.1.8. Epub 2016 Apr 21. PMID: 27144227; PMCID: PMC4853888.
- ↑ Jump up to: 14.0 14.1 Guntinas-Lichius O, Volk GF, Olsen KD, Mäkitie AA, Silver CE, Zafereo ME, Rinaldo A, Randolph GW, Simo R, Shaha AR, Vander Poorten V, Ferlito A. Facial nerve electrodiagnostics for patients with facial palsy: a clinical practice guideline. Eur Arch Otorhinolaryngol. 2020 Jul;277(7):1855-1874. doi: 10.1007/s00405-020-05949-1. Epub 2020 Apr 8. PMID: 32270328; PMCID: PMC7286870.
- ↑ Jump up to: 15.00 15.01 15.02 15.03 15.04 15.05 15.06 15.07 15.08 15.09 15.10 15.11 Harris BN, Tollefson TT. Facial reanimation: evolving from static procedures to free tissue transfer in head and neck surgery. Curr Opin Otolaryngol Head Neck Surg. 2015 Oct;23(5):399-406. doi: 10.1097/MOO.0000000000000193. PMID: 26339971.
- ↑ Jump up to: 16.0 16.1 16.2 16.3 16.4 Pinkiewicz M, Dorobisz K, Zatoński T. A Comprehensive Approach to Facial Reanimation: A Systematic Review. J Clin Med. 2022 May 20;11(10):2890. doi: 10.3390/jcm11102890. PMID: 35629016; PMCID: PMC9143601.
- ↑ Biglioli F, Bolognesi F, Allevi F, Rabbiosi D, Cupello S, Previtera A, Lozza A, Battista VMA, Marchetti C. Mixed facial reanimation technique to treat paralysis in medium-term cases. J Craniomaxillofac Surg. 2018 May;46(5):868-874. doi: 10.1016/j.jcms.2018.03.003. Epub 2018 Mar 14. PMID: 29625866.
- ↑ Ishii LE. Facial Nerve Rehabilitation. Facial Plast Surg Clin North Am. 2016 Nov;24(4):573-575. doi: 10.1016/j.fsc.2016.06.010. PMID: 27712822.
- ↑ House JW, Brackmann DE. Facial nerve grading system. Otolaryngol Head Neck Surg. 1985 Apr;93(2):146-7. doi: 10.1177/019459988509300202. PMID: 3921901.
- ↑ Jump up to: 20.0 20.1 20.2 20.3 20.4 20.5 Divi V, Deschler DG. Re-animation and rehabilitation of the paralyzed face in head and neck cancer patients. Clin Anat. 2012 Jan;25(1):99-107. doi: 10.1002/ca.21286. Epub 2011 Oct 24. PMID: 22025410
- ↑ Jump up to: 21.0 21.1 21.2 21.3 Ridgway JM, Bhama PK, Kim JH. Rehabilitation of facial paralysis. In: Cummings otolaryngology head and neck surgery, Vol III. 6th ed. Saunders; 2015; pp. 2643–2661 (Chapter 172).
- ↑ Jump up to: 22.0 22.1 Hadlock TA, Cheney ML, McKenna MJ. Facial reanimation surgery. In: Nadol Jr JP, Mckenna MJ, editors. Surgery of the ear and temporal bone.. Philadelphia (PA): Lippincott Williams and Wilkins; 2005. pp. 461–472.
- ↑ Bermudez LE, Nieto LE. Masseteric-facial nerve anastomosis: case report. J Reconstr Microsurg. 2004 Jan;20(1):25-30. doi: 10.1055/s-2004-818046. PMID: 14973772.
- ↑ Jump up to: 24.0 24.1 Wang W, Yang C, Li Q, et al. Masseter-to-facial nerve transfer: a highly effective technique for facial reanimation after acoustic neuroma resection. Ann Plast Surg 2014; 73:S63–S69.
- ↑ Klebuc MJA. Facial reanimation using the masseter-to-facial nerve transfer. Plast Reconstr Surg. 2011 May;127(5):1909-1915. doi: 10.1097/PRS.0b013e31820e9138. PMID: 21532419.
- ↑ Balaji SM. A modified temporalis transfer in facial reanimation. Int J Oral Maxillofac Surg. 2002 Dec;31(6):584-91. doi: 10.1054/ijom.2002.0254. PMID: 12521312.
- ↑ Jump up to: 27.0 27.1 Biglioli F, Zago M, Allevi F, Ciprandi D, Dell'Aversana Orabona G, Pucciarelli V, Rabbiosi D, Pacifici I, Tarabbia F, Sforza C. Reanimation of the paralyzed lids by cross-face nerve graft and platysma transfer. J Craniomaxillofac Surg. 2018 Mar;46(3):521-526. doi: 10.1016/j.jcms.2017.12.022. Epub 2017 Dec 27. PMID: 29311017.
- ↑ Jump up to: 28.0 28.1 Yoleri L, Songür E, Yoleri O, Vural T, Cağdaş A. Reanimation of early facial paralysis with hypoglossal/facial end-to-side neurorrhaphy: a new approach. J Reconstr Microsurg. 2000 Jul;16(5):347-55; discussion 355-6. doi: 10.1055/s-2000-7344. PMID: 10954315.
- ↑ Biglioli F, Colombo V, Rabbiosi D, Tarabbia F, Giovanditto F, Lozza A, Cupello S, Mortini P. Masseteric-facial nerve neurorrhaphy: results of a case series. J Neurosurg. 2017 Jan;126(1):312-318. doi: 10.3171/2015.12.JNS14601. Epub 2016 Apr 1. PMID: 27035172.
- ↑ Jump up to: 30.0 30.1 30.2 30.3 Mehta RP. Surgical treatment of facial paralysis. Clin Exp Otorhinolaryngol. 2009 Mar;2(1):1-5. doi: 10.3342/ceo.2009.2.1.1. Epub 2009 Mar 26. PMID: 19434284; PMCID: PMC2671829.
- ↑ Yetiser S. Total facial nerve decompression for severe traumatic facial nerve paralysis: a review of 10 cases. Int J Otolaryngol. 2012;2012:607359. doi: 10.1155/2012/607359. Epub 2011 Nov 20. PMID: 22164173; PMCID: PMC3228390.
- ↑ Jump up to: 32.0 32.1 32.2 Hasmat S, Cleary J, Suaning GJ, Lovell NH, Low TH, Clark JR. Dynamic facial reanimation using active implantable prosthesis: Restoring blink. J Plast Reconstr Aesthet Surg. 2021 Jul;74(7):1633-1701. doi: 10.1016/j.bjps.2021.01.001. Epub 2021 Jan 27. PMID: 33593711.
- ↑ Bobrov A, Batulin D, Shoferystov S, Popov A, Borysenko O. Implantable Closed-Loop System for Restoration of Blinking in Case of Unilateral Facial Nerve Paralysis. J Int Adv Otol. 2021 Sep;17(5):438-445. doi: 10.5152/iao.2021.21109. PMID: 34617896; PMCID: PMC8975379.
- ↑ Jump up to: 34.0 34.1 34.2 34.3 Langille M, Singh P. Static Facial Slings: Approaches to Rehabilitation of the Paralyzed Face. Facial Plast Surg Clin North Am. 2016 Feb;24(1):29-35. doi: 10.1016/j.fsc.2015.09.007. PMID: 26611699.
- ↑ Ayshford CA, Shykhon M, Uppal HS, Wake M. Endoscopic repair of nasal septal perforation with acellular human dermal allograft and an inferior turbinate flap. Clin Otolaryngol Allied Sci. 2003 Feb;28(1):29-33. doi: 10.1046/j.1365-2273.2003.00654.x. PMID: 12580877.
- ↑ Alex JC, Nguyen DB. Multivectored suture suspension: a minimally invasive technique for reanimation of the paralyzed face. Arch Facial Plast Surg. 2004 May-Jun;6(3):197-201. doi: 10.1001/archfaci.6.3.197. PMID: 15148131.
- ↑ Jump up to: 37.0 37.1 37.2 37.3 37.4 37.5 37.6 Morisada, M. V., Hoshal, S. G., & Tollefson, T. T. (2021). Periorbital reconstruction in facial paralysis. Operative Techniques in Otolaryngology-Head and Neck Surgery, 32(4), 213–218. https://doi.org/10.1016/j.otot.2021.10.012
- ↑ Jump up to: 38.0 38.1 38.2 38.3 38.4 Oh TS, Min K, Song SY, Choi JW, Koh KS. Upper eyelid platinum weight placement for the treatment of paralytic lagophthalmos: A new plane between the inner septum and the levator aponeurosis. Arch Plast Surg. 2018 May;45(3):222-228. doi: 10.5999/aps.2017.01599. Epub 2018 May 15. PMID: 29788690; PMCID: PMC5968324.
- ↑ Jump up to: 39.0 39.1 39.2 Guy, C. L., & Ransohoff, J. (1968). The Palpebral Spring for Paralysis of the Upper Eyelid in Facial Nerve Paralysis: Technical Note. Journal of Neurosurgery, 29(4), 431-433. https://doi.org/10.3171/jns.1968.29.4.0431
- ↑ Jump up to: 40.0 40.1 40.2 40.3 Rajak S, Rajak J, Selva D. Performing a tarsorrhaphy. Community Eye Health. 2015;28(89):10-1. PMID: 26435586; PMCID: PMC4579993.
- ↑ Patel BC, Malhotra R. Upper Eyelid Blepharoplasty. [Updated 2023 Jul 24]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK537078/
- ↑ Ducic Y, Adelson R. Use of the endoscopic forehead-lift to improve brow position in persistent facial paralysis. Arch Facial Plast Surg. 2005 Jan-Feb;7(1):51-4. doi: 10.1001/archfaci.7.1.51. PMID: 15655175.
- ↑ Servat, J. J., Black, E. H., Mishulin, A., Gladstone, G. J., Scott, I. U., & Fekrat, S. (2016, March 21). How to perform an endoscopic forehead and eyebrow lift. American Academy of Ophthalmology. https://www.aao.org/eyenet/article/how-to-perform-endoscopic-forehead-eyebrow-lift
- ↑ Jump up to: 44.0 44.1 Jue MS, Yoo J, Kim MS, Park HJ. The Lateral Tarsal Strip for Paralytic Ectropion in Patients with Leprosy. Ann Dermatol. 2017 Dec;29(6):742-746. doi: 10.5021/ad.2017.29.6.742. Epub 2017 Oct 30. PMID: 29200763; PMCID: PMC5705356.
- ↑ Jump up to: 45.0 45.1 45.2 45.3 Dedhia, R. D., Shipchandler, T. Z., & Tollefson, T. T. (2021). Eyelid coupling using a modified tarsoconjunctival flap in facial paralysis. Facial Plastic Surgery Clinics of North America, 29(3), 447–451. https://doi.org/10.1016/j.fsc.2021.03.007
- ↑ Jump up to: 46.0 46.1 de Sanctis Pecora C, Shitara D. Botulinum Toxin Type A to Improve Facial Symmetry in Facial Palsy: A Practical Guideline and Clinical Experience. Toxins (Basel). 2021 Feb 18;13(2):159. doi: 10.3390/toxins13020159. PMID: 33670477; PMCID: PMC7923088.
- ↑ Hammerschlag PE. Facial reanimation with jump interpositional graft hypoglossal facial anastomosis and hypoglossal facial anastomosis: evolution in management of facial paralysis. Laryngoscope. 1999 Feb;109(2 Pt 2 Suppl 90):1-23. doi: 10.1097/00005537-199902001-00001. PMID: 10884169.
- ↑ Jump up to: 48.0 48.1 48.2 48.3 Matos Cruz AJ, Hohman MH, De Jesus O. Facial Nerve Repair. 2023 Aug 23. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan–. PMID: 32809458.
- ↑ Sánchez-Ocando M, Gavilán J, Penarrocha J, González-Otero T, Moraleda S, Roda JM, Lassaletta L. Facial nerve repair: the impact of technical variations on the final outcome. Eur Arch Otorhinolaryngol. 2019 Dec;276(12):3301-3308. doi: 10.1007/s00405-019-05638-8. Epub 2019 Sep 19. PMID: 31538238.
- ↑ Prasad SC, Balasubramanian K, Piccirillo E, Taibah A, Russo A, He J, Sanna M. Surgical technique and results of cable graft interpositioning of the facial nerve in lateral skull base surgeries: experience with 213 consecutive cases. J Neurosurg. 2018 Feb;128(2):631-638. doi: 10.3171/2016.9.JNS16997. Epub 2017 Apr 7. PMID: 28387625.
- ↑ Malik TH, Kelly G, Ahmed A, Saeed SR, Ramsden RT. A comparison of surgical techniques used in dynamic reanimation of the paralyzed face. Otol Neurotol. 2005 Mar;26(2):284-91. doi: 10.1097/00129492-200503000-00028. PMID: 15793421.
- ↑ Rashid HU, Rehman IU, Rashid M, Yousaf S, Khan N, Aqsa A. Results Of Immediate Facial Nerve Reconstruction In Patients Undergoing Parotid Tumour Resection. J Ayub Med Coll Abbottabad. 2019 Jul-Sep;31(3):340-345. PMID: 31535502.
- ↑ Mohamed A., Omi E., Honda K., Suzuki S., Ishikawa K. Outcome of different facial nerve reconstruction techniques. Braz. J. Otorhinolaryngol. 2016;82:702–709. doi: 10.1016/j.bjorl.2015.12.010.
- ↑ Shindo M. Management of facial nerve paralysis. Otolaryngol Clin North Am. 1999 Oct;32(5):945-64. doi: 10.1016/s0030-6665(05)70183-3. PMID: 10477797.
- ↑ Bianchi B, Ferri A, Ferrari S, Copelli C, Magri A, Ferri T, Sesenna E. Cross-facial nerve graft and masseteric nerve cooptation for one-stage facial reanimation: principles, indications, and surgical procedure. Head Neck. 2014 Feb;36(2):235-40. doi: 10.1002/hed.23300. Epub 2013 Jun 1. PMID: 23728740.