Ectasia Risk in Topography
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
LASIK and PRK induced ectasia is preventable if the preoperative corneal topography/tomography and pachymetry indexes for high-risk are identified on time.[1][2][3]
Disease Entity
Refractive surgery induced ectasia is probably the most feared complication of laser in situ keratomileusis (LASIK), photorefractive keratectomy (PRK), and small incision lenticule extraction (SMILE). It presents as a progressive eccentric thinning of the corneal stroma with consequent steepening of the anterior and posterior surface of the cornea. The disorder is considered irreversible and may reduce significantly uncorrected and spectacle-corrected visual acuity.[3] Although the general prevalence of postoperative ectasia is unknown, there are several reports that show a prevalence rate that vary from 0.02% to 0.6% .[4][5] Risk factors for the development of post-LASIK/PRK ectasia include a personal or family history of keratoconus (KC), abnormal corneal topography/tomography (form fruste keratoconus), high myopia, low-residual stromal bed (RSB <300 microns), excessive stromal ablation (>100 microns), high percentage of tissue altered (PTA >0.40), deep primary keratotomy resulting in a thick corneal flap and low preoperative corneal thickness (<500 microns).[3] [6][7][8]
Even when risk factors for ectasia after refractive surgery have been identified, reports of individuals developing ectasia without any of the proposed risk factors have emerged - this specific group of patients tend to be younger.[3][9][10][11] Eye rubbing could explain the ectasia occurrence in some cases without other identified risk factors.
Etiology
Corneal ectasia induced by excimer laser refractive surgery is thought to be the result of the alteration of the corneal surface and anterior stroma in corneas with previous biomechanical abnormalities.[12] The prevalence is between 0.02 to 0.06%.[13][14] Histologic and ultrastructural findings explain the pathophysiology of post-LASIK or post-PRK ectasia by interlamellar and interfibrillar biomechanics slippage induced by excimer laser disruption (interlamelar and interfibrillar fracture) and not to primary collagen fibrillar failure.[12] Besides geometric corneal parameters, new techniques permit a better understanding of the corneal bio-mechanics in order to get a better prediction of the corneal response to surgical or therapeutic interventions and to assist in the detection of early KC. [15][16][17]
Diagnosis
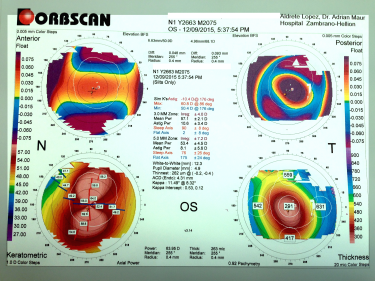
Post-refractive ectasia is often suspected in patients with progressive myopia or astigmatism after refractive surgery. This can occur as soon as months or as late as years after refractive surgery. A careful history, with emphasis on patient's pre-surgery parameters, can help with suspecting post-refractive ectasia. In advanced cases, the patients often appear similar to keratoconus. Topography is often used to diagnose post-refractive ectasia, displaying inferior steepening, thin corneas, and abnormal posterior elevation - findings similar to keratoconus.
In 2015, a group of international cornea specialists gathered in order to define key points in the global consensus on keratoconus and ectatic diseases.[18] “Ectasia progression” by this group was defined by a consistent change in at least 2 of the following parameters where the magnitude of the change is above the normal noise of the testing system:
- Steepening of the anterior corneal surface
- Steepening of the posterior corneal surface
- Thinning and/or an increase in the rate of corneal thickness change from the periphery to the thinnest point.
The group noted that the changes need to be consistent over time and above the normal variability of the testing system. They also noted that while progression often is associated with loss of uncorrected and best spectacle corrected visual acuity (BSCVA), this is not necessary. The panel recommended that interval between testing be shorter for younger patients and that the same machine be used for testing as much as possible.
Risk Factors
Because post-refractive ectasia is irreversible and can lead to permanent vision loss, much of the focus in refractive surgery is prevention and adequate screening of patients presenting for refractive surgery evaluations. It has been estimated that up to 6% of patients with some sort of pre-clinically ectatic condition show up in clinical settings for refractive evaluation. [19][20][21] While there are various strategies in identifying high risk patients, the combination of abnormal corneal topography and altered corneal tissue after surgery help stratify risk in patients.[22][23][24][25][26][27][28][29][30][31]
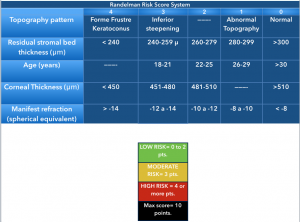
Randleman et al. compared normal uneventful LASIK patients with those developing corneal ectasia after LASIK or PRK and observed that the latter had significant abnormal preoperative corneal topographies, were younger (< 34 years), were more myopic (>8 diopters of mean refractive spherical equivalent), had thinner corneas before surgery (mean, 521 microns) and had less RSB thickness (256 microns).[3] Logistic regression analysis on their data showed abnormal topography as the most significant factor that discriminated cases from controls. [1] [3] A screening strategy that weighs all of these factors, the Ectasia Risk Scoring System, was proposed and a score between 0 to 4 was assigned to each one of the factors[1].
- Abnormal preoperative topography
- Low Residual Stromal Bed (RSB) thickness
- Young age
- Low preoperative corneal thickness
- High myopia
The scoring is a useful but still controversial index for preoperative LASIK candidates.
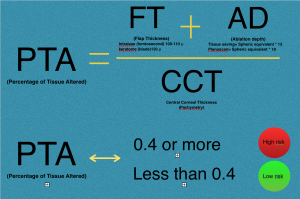
Santiago et al, found that the percent of tissue altered (PTA) ≥40% during LASIK (derived from [PTA = (FT + AD)/CCT] where FT is flap thickness, AD is ablation depth, and CCT is central corneal thickness) is strongly associated with the development of ectasia in eyes with otherwise normal topography.[22] The percent of tissue altered (PTA) describes the interaction between flap thickness (FT), the ablation depth (AD) and the central corneal thickness (CCT) during excimer laser refractive surgery. This finding was challenged by other reports that did not find a higher occurrence of ectasia in cases with a PTA > 40% and normal topography and tomography. [32][33][34][35]
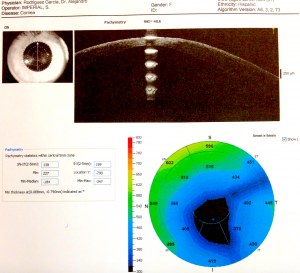
Tomography and topography can also help with screening for risk factors. Topography is often placido disk-based and can help image the anterior corneal surface. Tomography presents a 3D view of the cornea and can help assess anterior and posterior corneal surface, as well as providing pachymetric mapping of the entire cornea. It can also help assess epithelial thickness mapping. The classic features of keratoconus, such as high keratometry (>47D), focal steepening, inferior-superior ratio asymmetry (>1.4D), and posterior elevation can suggest poor candidates for refractive surgery.
Belin-Ambrosio Enhanced Ectasia Display software (available in the Pentacam SScheimpflug tomography) analyses 9 parameters ( Df (front anterior elevation change), Db (back posterior elevation change), Dp (pachymetric progression), Dt (thinnest point pachymetry), Dy (displacement of the thinnest point), Ambrósio relational thickness maximum (ART-Max), anterior elevation, posterior elevation, and Kmaximum) to provide a "D value" to identify patients suspicious for keratoconus, who would be poor candidates for refractive surgery.[36]
Epithelial thickness mapping may also be useful in screening for risk of ectasia - patients with keratoconus have a donut pattern of epithelial remodeling, consistent with a pattern of central epithelial thinning, with a ring of epithelial thickening.[37]
The Keratoconus Prediction Index (KPI), a system developed by Maeda and Klyce, is able to differentiate keratoconus from a wide variety of other pathologies with a false positive rate of 98.7% and a false negative rate of 98.4%.[38]
Saad and Gatinel combined topographic and tomographic parameters to create an automated scoring system (Score Analyser) on a slit scanning tomographer[39] able to detect forme fruste keratoconus with 92% accuracy.
Down syndrome, family history of connective tissue disorders and specifically keratoconus, ocular allergy, and mechanical factors such as eye rubbing and floppy eyelid syndrome, have also been identified as risk factors for corneal ectasia. [18][40] Pointing out a patient with FFK and early KC is of major importance in the preoperative assessment, as most refractive procedures are contraindicated in patients with an ectatic preoperative condition due to the high-risk of induction and/or progression of the ectasia after the procedure.[41]
Genetic testing has been used as part of the screening modality, especially in patients with features that may be considered high risk for refractive surgery. DNA testing by Avellino Labs were FDA-approved in 2021 to help elucidate patients at risk for ectasia. Its clinical utility and broad applicability is still being elucidated.[42]
Pathophysiology
Electron microscopic studies show Z-shaped pattern interruptions at the level of Bowman´s layer that are thought to have been caused by breaks in the epithelial layer, with posterior extension of the epithelial layer into Bowman´s layer and collagen extending anteriorly into the epithelial layer.[43] The fragmentation observed in the Bowman´s layer with the electron microscopy scanner tend to be similar to keratoconus, and it is believed to be the initial change that then leads to ectasia.[44]
The basis of the histologic and ultra-structural findings proposed by Dawson et al.[45] in the pathophysiology of post-LASIK or post-PRK ectasia is explained by interlamellar bio-mechanical slippage followed by subsequent interfibrillar bio-mechanical slippage as opposed to direct primary collagen fibril failure. This chronic bio-mechanical failure process is similar to that of interlamellar and interfibrillar slippage described with keratoconus.[46][47][48][49]
An atypical histopathogical variant has been described, where breaks in Bowman´s layer are not present, although the patient clinically presents like post-refractive ectasia. Among different histopathologic variants, the scarring of Bowman´s layer and anterior stroma positively correlate with fragmentation of collagen fibers and fibroblastic activity.[44][50]
History
Marc Amsler In 1938, with a photographic placido disk, was the first to describe early corneal topographic changes in keratoconus before clinical or bio-microscopic signs could be noted. His classical studies of keratoconus documented the progression of the disease, from minor corneal surface changes to clinically identifiable keratoconus. He classified keratoconus into clinical stages and an earlier latent stage identifiable only with the placido disk evaluation of corneal topography.
Amsler reexamined 286 eyes 3–8 years after the diagnosis, and only 20%, including 66% of the latent cases, show progression. Progression was most likely to occur in patients between 10 and 20 years of age and decreased tendency to progression between ages 20 and 30, and low incidence of progression after 30 years old.[51]
In the 1970s, Donaldson published a new technique for keratography: The photokeratoscope produces a topographic record of 55–80% of the total corneal contour, but it provides little information about the central 2-3 mm of the cornea.[52] Rowsey et al. used this instrument to study keratoconus in 827 patients.[53]
The ophthalmometer or keratometer provides information from 3 points approximately 3 mm apart, calculating anterior corneal curvature. This was a simple and useful tool for detecting keratoconus.
Krachmer et al. documented that increasing corneal curvature over time using the keratometer is a sensitive indicator of keratoconus.[54]
In 1984 the classic publication of Klyce et al. set the basis for the later development of computer-assisted videokeratoscopes algorithms. The computed process was a useful quantitative method with which to determine corneal shape for clinical evaluation, particularly in corneal surface irregularities.[55]
Current corneal imaging modalities such as Scheimpflug and optical coherence tomography (OCT) offer more information than were previously available with placido based anterior corneal analysis. This new tomographic information allows for earlier identification of disease and has changed our perception of what constitutes keratoconus, improving the specificity to exclude false positive cases.[56] OCT and Scheimpflug photography allows measurements of both the anterior and posterior cornea and corneal thickness maps, providing greater corneal coverage than possible with videokeratoscopes.[57] Of these, rotating Scheimpflug photography currently provides the most useful information for diagnosing early ectatic change. It is also a technique that is rapid and easy to perform, which would be a requisite for use as a screening tool.[58][59][56]
Differential diagnosis
Meticulous slit lamp examination may distinguish from keratoglobus, pellucid marginal degeneration and Terrien marginal degeneration. But topographic evidence is necessary in early presentations or non typical cases.[60]
Pellucid Marginal Degeneration.
Typically described by a peripheral band of thinning of the inferior cornea, there is an uninvolved area between the thin corneal area and sclero-corneal limbus. Central corneal thickness is usually normal. Pellucid is a progressive condition affecting both eyes, though can be asymmetric. The topographic pattern of the cornea classically resemble a butterfly, with against the rule astigmatism. [21]
Terrien Marginal degeneration
Terrien marginal degeneration is a slow-progressing, bilateral asymmetric degeneration of the peripheral cornea. Males over 40 years are more commonly affected. Stromal thinning, vascularization, lipid deposition, and against-the-rule astigmatism are classic signs. Though typically noninflammatory, a variant form characterized by prominent inflammation exists. [61]
Keratoglobus
Keratoglobus is a rare bilateral disorder in which the entire cornea is thinned most markedly near the corneal limbus, in contrast to the localized thinning centrally or paracentrally in keratoconus. [54] Most often keratoglobus is a congenital disease, but it may be secondarily acquired. This corneal pathology tend to produce extreme myopia, irregular astigmatism, and corneal scarring due to hydrops.[62]
Additional Resources[63]
- American Academy of Ophthalmology
- National Keratoconus Foundation NKCF
- National Eye Institute
- Clinical trials for corneal diseases
- Ectasia After LASIK
References
- ↑ Jump up to: 1.0 1.1 1.2 Randleman JB, Trattler WB, Stulting RD. Validation of the Ectasia Risk Score System for Preoperative Laser in Situ Keratomileusis Screening. American journal of ophthalmology. 2008;145(5):813-818. doi:10.1016/j.ajo.2007.12.033.
- ↑ Randleman JB, Russell B, Ward MA, Thompson KP, Stulting RD. Risk factors and prognosis for corneal ectasia after LASIK. Ophthalmology. 2003;110:267–75. [PubMed]
- ↑ Jump up to: 3.0 3.1 3.2 3.3 3.4 3.5 Randleman JB, Woodward M, Lynn MJ, Stulting RD. Risk Assessment for Ectasia after Corneal Refractive Surgery. Ophthalmology. 2008;115:37–50. [PubMed]
- ↑ Binder PS. Analysis of ectasia after laser in situ keratomileusis: risk factors. J Cataract Refract Surg 2007; 33(9):1530–1538.
- ↑ Chen MC, Lee N, Bourla N, Hamilton DR. Corneal biomechanics measurements before and after laser in situ keratomileusis. J Cataract Refract Surg 2008; (34)11:1886–1891.
- ↑ Ortiz D, Piñero D, Shabayek MH, Arnalich-Montiel F, Alió JL. Corneal biomechanical properties in normal, post-laser in situ keratomileusis, and keratoconic eyes. Journal of Cataract and Refractive Surgery. 2007;33(8):1371–1375.
- ↑ González-Méijome JM, Villa-Collar C, Queirós A, Jorge J, Parafita MA. Pilot study on the influence of corneal biomechanical properties over the short term in response to corneal refractive therapy for myopia. Cornea. 2008;27(4):421–426.
- ↑ Kirwan C, O’Keefe M. Corneal hysteresis using the Reichert ocular response analyser: findings pre- and post-LASIK and LASEK. Acta Ophthalmologica. 2008;86(2):215–218.
- ↑ Argento C, Cosentino MJ, Tytiun A, et al. Corneal ectasia after laser in situ keratomileusis. J Cataract Refract Surg 2001;27:1440–8.
- ↑ Wang JC, Hufnagel TJ, Buxton DF. Bilateral keratectasia after unilateral laser in situ keratomileusis: a retrospective diagnosis of corneal ectasia . J Cataract Refract Surg 2003;29:2015–8
- ↑ Piccoli PM, Gomes AA, Piccoli FV. Corneal ectasia detected 32 months after LASIK for correction of myopia and asymmetric astigmatism. J Cataract Refract Surg 2003;29:1222–5.
- ↑ Jump up to: 12.0 12.1 Dawson DG1, Randleman JB, Grossniklaus HE, O'Brien TP, Dubovy SR, Schmack I, Stulting RD, Edelhauser HF. Corneal ectasia after excimer laser keratorefractive surgery: histopathology, ultrastructure, and pathophysiology.Ophthalmology 2008;115(12):2181-2191
- ↑ Lifshitz T, Levy J, Klemperer I, Levinger S. Late bilateral keratectasia after LASIK in a low myopic patient. J Refract Surg 2005;21:494–496
- ↑ Seiler T, Quurke AW. Iatrogenic keratectasia after LASIK in a case of forme fruste keratoconus. J Cataract Refract Surg 1998;24:1007–1009
- ↑ Garcia-Porta, N., Fernandes, P., Queiros, A., Salgado-Borges, J., Parbfita-Mato, M., & González-Méijome, J. M. (2014). Corneal Biomechanical Properties in Different Ocular Conditions and New Measurement Techniques. ISRN Ophthalmology, 2014, 724546. http://doi.org/10.1155/2014/724546
- ↑ Luce DA. Determining in vivo biomechanical properties of the cornea with an Ocular Response Analyzer. Journal of Cataract and Refractive Surgery. 2005;31(1):156–162.
- ↑ Piñero DP, Alcón N. In vivo characterization of corneal biomechanics.J Cataract Refract Surg. 2014 Jun;40(6):870-87.
- ↑ Jump up to: 18.0 18.1 Gomes JA, Tan D, Rapuano CJ, Belin MW, Ambrósio R Jr, Guell JL, Malecaze F, Nishida K, Sangwan VS;Global consensus on keratoconus and ecstatic diseases. Group of Panelists for the Global Delphi Panel of Keratoconus and Ectatic Diseases. Cornea. 2015 Apr;34(4):359-69. doi: 10.1097/ICO.0000000000000408. PMID: 25738235
- ↑ Nesburn AB, Bahri S, Salz J, et al: Keratoconus detected by videokeratography in candidates for photorefractive keratectomy. J Refract Surg 11:194–201, 1995
- ↑ Wilson SE, Klyce SD: Screening for corneal topographic abnormalities before refractive surgery. Ophthalmology 101:147–152, 1994
- ↑ Jump up to: 21.0 21.1 Rabinowitz, Yaron S. Keratoconus. Survey of Ophthalmology, Volume 42, Issue 4, 297 - 319
- ↑ Jump up to: 22.0 22.1 Santhiago MR, Smadja D, Gomes BF, Mello GR, Monteiro ML, Wilson SE, Randleman JB. Association between the percent tissue altered and post-laser in situ keratomileusis ectasia in eyes with normal preoperative topography. Am J Ophthalmol. 2014 Jul;158(1):87-95.e1. doi: 10.1016/j.ajo.2014.04.002. Epub 2014 Apr 13. PubMed PMID: 24727263.
- ↑ Santhiago MR, Wilson SE, Hallahan KH, et al. Changes in custom biomechanical variables after femtosecond laser in situ keratomileusis and photorefractive keratectomy for myopia. J Cataract Refract Surg. http://dx.doi.org/10.1016/j.jcrs.2013.11.030.
- ↑ Smadja D, Santhiago MR, Mello GR, Roberts CJ, Dupps WJ Jr, Krueger RR. Response of the posterior corneal surface to myopic laser in situ keratomileusis with different ablation depths. J Cataract Refract Surg 2012;38(7): 1222–1231.
- ↑ Reinstein DZ, Archer TJ, Randleman JB. Mathematical model to compare the relative tensile strength of the cornea after PRK, LASIK, and small incision lenticule extraction.J Refract Surg 2013;29(7):454–460.
- ↑ Randleman JB, Hebson CB, Larson PM. Flap thickness in eyes with ectasia after laser in situ keratomileusis. J Cataract Refract Surg 2012;38(5):752–757.
- ↑ Saad A, Gatinel D. Bilateral corneal ectasia after laser in situ keratomileusis in patient with isolated difference in central corneal thickness between eyes. J Cataract Refract Surg 2010;36(6):1033–1035.
- ↑ Seiler T, Koufala K, Richter G. Iatrogenic keratectasia after laser in situ keratomileusis. J Refract Surg 1998;14:312–7.
- ↑ Seiler T, Quurke AW. Iatrogenic keratectasia after LASIK in a case of forme frusta keratoconus. J Cataract Refract Surg 1998;24:1007–9.
- ↑ Buzard KA, Tuengler A, Febbraro JL. Treatment of mild to moderate keratoconus with laser in situ keratomileusis. J Cataract Refract Surg 1999;25:1600 –9.
- ↑ Geggel HS, Talley AR. Delayed onset corneal ectasia following laser in situ keratomileusis. J Cataract Refract Surg 1999;25: 582–6.
- ↑ Saad A, Binder PS, Gatinel D. Evaluation of the percentage tissue altered as a risk factor for developing post-laser in situ keratomileusis ectasia. J Cataract Refract Surg. 2017 Jul;43(7):946-951.
- ↑ Yao-Wen Song 1, Rui He, Jack X Ma, Douglas D Koch, Li Wang. Long-term safety of laser in situ keratomileusis in eyes with thin corneas: 5-year follow-up. Int J Ophthalmol. 2018 Jul 18;11(7):1227-1233.
- ↑ Maja Bohac, Mateja Koncarevic, Adi Pasalic, Alma Biscevic, Maja Merlak, Nikica Gabric, Sudi Patel. Incidence and Clinical Characteristics of Post LASIK Ectasia: A Review of over 30,000 LASIK Cases. Semin Ophtalmo 2018;33(7-8):869-877.
- ↑ Gatinel D, Saad A, Binder PS. Comparison of the effect of LASIK parameters on the percent tissue altered (1-dimensional metric) versus percent volume altered (3-dimensional metric). J Cataract Refract Surg. 2018 Jul;44(7):897-904.
- ↑ O’hEineachain R. The BAD may be better for detecting ectatic disease and its susceptibility. EuroTimes Cornea Update 2010
- ↑ Franco J, White CA, Kruh JN. Analysis of Compensatory Corneal Epithelial Thickness Changes in Keratoconus Using Corneal Tomography. Cornea. Mar 2020;39(3):298-302. doi:10.1097/ico.0000000000002156
- ↑ Maeda, N, Klyce, SD, and Smolek, MK. Comparison of methods for detecting keratoconus using videokeratography. Arch Ophthalmol. 1995; 113: 870–874
- ↑ Saad A, Gatinel DTopographic and tomographic properties of forme fruste keratoconus corneas. Invest Ophthalmol Vis Sci. 2010 Nov;51(11):5546-55
- ↑ Mazharian A, Panthier C, Courtin R, Jung C, Rampat R, Saad A, Gatinel D.Incorrect sleeping position and eye rubbing in patients with unilateral or highly asymmetric keratoconus: a case-control study. Graefes Arch Clin Exp Ophthalmol. 2020 Nov;258(11):2431-2439
- ↑ Lafond G, Bazin R, Lajoie C. Bilateral severe keratoconus after laser in situ keratomileusis in a patient with forme fruste keratoconus. J Cataract Refract Surg. 2001 Jul;27(7):1115-8. PubMed PMID: 11489585.
- ↑ McMonnies CW. Screening for keratoconus suspects among candidates for refractive surgery. Clinical & experimental optometry. Nov 2014;97(6):492-8. doi:10.1111/cxo.12169
- ↑ Kanai A, Kaufman HE. Electron microscopic studies of corneal stroma: aging changes of collagen fibers. Ann Ophthalmol. 1973 Mar;5(3):285-7 passim. PubMed PMID: 4694913.
- ↑ Jump up to: 44.0 44.1 Sawaguchi S, Fukuchi T, Abe H, Kaiya T, Sugar J, Yue BY. Three-dimensional scanning electron microscopic study of keratoconus corneas. Arch Ophthalmol. 1998 Jan;116(1):62-8. PubMed PMID: 9445209.
- ↑ Dawson DG, Randleman JB, Grossniklaus HE, O'Brien TP, Dubovy SR, Schmack I, Stulting RD, Edelhauser HF. Corneal ectasia after excimer laser keratorefractive surgery: histopathology, ultrastructure, and pathophysiology. Ophthalmology. 2008 Dec;115(12):2181-2191.e1. doi: 10.1016/j.ophtha.2008.06.008. Epub 2008 Aug 9. PubMed PMID: 18692245.
- ↑ Bron AJ. The architecture of the corneal stroma. Br J Ophthalmic 2001;85:379–81.
- ↑ Bron AJ. Keratoconus. Cornea 1988;7:163–9.
- ↑ Patey A, Salvoldelli M, Pouliquen Y. Keratoconus and normal cornea: a comparative study of the collagenous fibers of the corneal stroma by image analysis. Cornea 1984;3:119–24
- ↑ Polack FM. Contributions of electron microscopy to the study of corneal pathology. Surv Ophthalmol. 1976 May-Jun;20(6):375-414. Review. PubMed PMID: 779087.
- ↑ Pouliquen Y, Graf B, Hamada R, Giraud JP, Offret G. [Fibrocytes in keratoconus. Morphological aspect and modification of the extra-cellular space. Study with light and electron microscopy]. Arch Ophtalmol Rev Gen Ophtalmol. 1972 Aug-Sep;32(8):571-86. French. PubMed PMID: 4267199.
- ↑ Amsler, M. Le keratocone fruste au javal. Ophthalmologica. 1938; 96: 77–83
- ↑ Donaldson, DD. A new instrument for keratography. Arch Ophthalmol. 1972; 88: 425–428
- ↑ Rowsey, JJ, Reynolds, AE, and Brown, R. Corneal topography. Corneascope. Arch Ophthalmol. 1981; 99: 1093–1100
- ↑ Jump up to: 54.0 54.1 Krachmer, JH, Feder, RS, and Belin, MW. Keratoconus and related noninflammatory corneal thinning disorders. Surv Ophthalmol. 1984; 28: 293–322
- ↑ Klyce, SD. Computer-assisted corneal topography. High resolution graphic presentation and analysis of keratoscopy. Invest Ophthalmol Vis Sci. 1984; 25: 1426–1435
- ↑ Jump up to: 56.0 56.1 Belin MW, Ambrósio R. Scheimpflug imaging for keratoconus and ectatic disease. Indian Journal of Ophthalmology. 2013;61(8):401-406. doi:10.4103/0301-4738.116059.
- ↑ Kim SW, Sun HJ, Chang JH, Kim EK, Anterior segment measurements using Pentacam and Orbscan II 1 to 5 years after refractive surgery. J Refract Surg. 2009 Dec; 25(12):1091-7.
- ↑ Yazici AT, Bozkurt E, Alagoz C, Alagoz N, Pekel G, Kaya V, Yilmaz OF, Central corneal thickness, anterior chamber depth, and pupil diameter measurements using Visante OCT, Orbscan, and Pentacam. J Refract Surg. 2010 Feb; 26(2):127-33.
- ↑ Belin MW, Khachikian SS. Corneal diagnosis and evaluation with the OCULUS pentacam. Highlights Ophthalmol. 2007;35:5–7.
- ↑ Rabinowitz YS, Wilson SE, Klyce SD (eds): Corneal Topography: Interpreting Videokeratography. New York, Tokyo, Ig- aku Shoin, 1993
- ↑ Chan AT, Ulate R, Goldich Y, Rootman DS, Chan CC. Terrien Marginal Degeneration: Clinical Characteristics and Outcomes. Am J Ophthalmol. 2015 Nov;160(5):867-872.e1. doi: 10.1016/j.ajo.2015.07.031. Epub 2015 Jul 23. PubMed PMID: 26210866.
- ↑ Baillif S, Garweg JG, Grange JD, Burillon C, Kodjikian L. [Keratoglobus: review of the literature]. J Fr Ophtalmol. 2005 Dec;28(10):1145-9. Review. French. PubMed PMID: 16395211.
- ↑ Asbell, P., & Petratos, T. (2015, August 28). Keratoconus (L. Rose, Ed.). Retrieved December 26, 2015, from https://eyewiki.org/Keratoconus