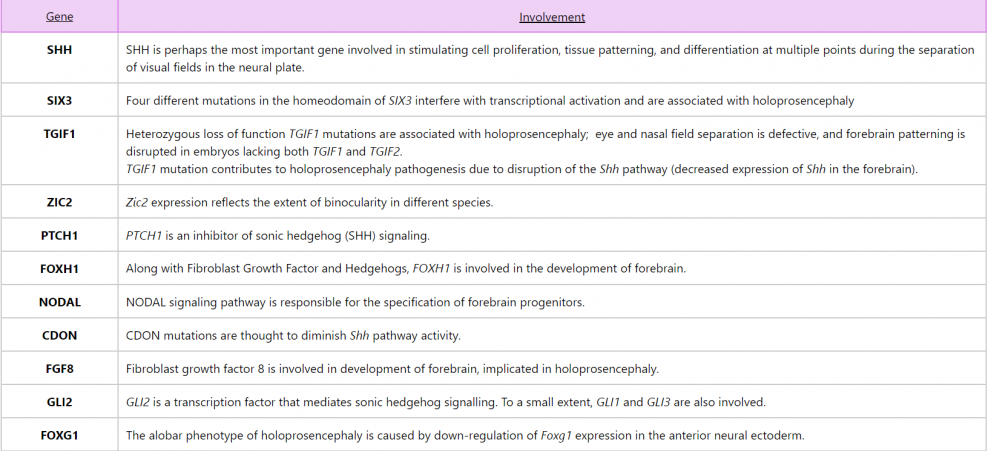
Cyclopia
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
A rare manifestation of the most severe degree of alobar holoprosencephaly, cyclopia, results when the eye fields fail to separate and develop as a single socket in the middle of the face with varying degrees of fusion of the globes. Since it is associated with very high neonatal mortality rates, miscarriages, and stillbirths; cyclopia is generally not encountered in clinical practice.
Disease Entity
Cyclopia. ICD-10 Reportable Congenital Malformations Coding : Q87.0
Disease
Cyclopia (also known as synophthalmia or cyclocephaly) is a clinical abnormality where fusion of both eyes can be seen in a single, central orbit located in the middle of the face. It is the facial expression of the most severe form of a congenital anomaly called holoprosencephaly syndrome (HPE).
Incidence
Synophthalmia, as a spectrum, occurs in 1/100,000 live births, out of which there is a female predominance of 58%[2]. The preponderance of female babies born with cyclopia may be explained by an increased number of male stillbirths with this anomaly.
Risk Factors
The etiology of cyclopia is still largely unknown but potential heterogeneous risk factors have been identified[3], some with stronger evidence than others.
Fetal factors:
- Chromosomal anomalies - most commonly associated with Patau syndrome (trisomy 13)
- Female sex
- Multiple pregnancies, especially twinning[4]
- Syndromic associations of alobar holoprosencephaly (eg. : Smith - Lemli - Opitz syndrome)
Maternal factors:
- Previous unexplained miscarriages
- Gestational diabetes
- TORCH complex / trans-placental infections
- Insulin (during the gestational period)
- Alcohol consumption
- Smoking (nicotine exposure)
- Retinoic acid exposure during pregnancy
- Anticonvulsant usage during pregnancy
- Lithium
- Birth control pills (to a smaller extent)
- Cyclopamine - a highly alkaloid toxin, Veratrum californicum, found in corn lily or false hellebore is implicated in causing cyclopia (when ingested believing the plant to be hellebore, which can cure the morning sickness symptoms associated with the first few months of pregnancy)
Pathophysiology
To understand how an anomaly like cyclopia occurs, we first need to have an understanding of the normal development of the neural tube, forebrain, and subsequently, the eye fields.
Once the notochord has been formed in the mesoderm by the invagination of primitive streak cells, it induces the neural ectoderm lying above to form the neural plate. The process of neurulation, or the conversion of the neural plate into the neural tube, is central to the development of the central and peripheral nervous system. In neurulation the neural plate folds over itself to form a tube, and now lies beneath the ectoderm and above the notochord. The cells in the area of fusion of the two ends of the neural plate, called the neural crest cells, come to lie directly above the neural tube, to later differentiate into most of the structures of the peripheral nervous system.
At this stage of development where neurulation has occurred, the hollow neural tube extends along the length of the embryo, and is the precursor of the brain and spinal cord. The part of the neural tube destined to be the future brain becomes three distinct dilated demarcations, called primary vesicles. The vesicles are given names corresponding to which part of the neural tube they occupy; from cranial to caudal - prosencephalon, mesencephalon, and rhombencephalon. These areas correspond, respectively, to the forebrain, midbrain, and the hindbrain. The remainder of the neural tube later becomes the spinal cord.
Additionally, the anterior-most part of the developing neural tube undergoes folding, so that the prosencephalon comes to lie ventral to the rhombencephalon, with the mesencephalon connecting the two. The primary vesicles differentiate further. The cranial-most part of the prosencephalon divides into two lateral vesicles, called the telencephalon, which will become the cerebrum. The remaining part of the prosencephalon is called the diencephalon, which will become the thalamus, epithalamus, and hypothalamus. The newly formed diencephalon and telencephalon are the secondary vesicles. Of particular interest in the discussion of cyclopia is the diencephalon, which is the area where the optic vesicles develop.
Around day 22, when the developing embryo is about 2 mm in length and is at the eight-somite stage, two small grooves develop on the sides of the developing forebrain. With the closure of the neural tube, these grooves become out-pouchings from the area of the primary brain vesicle, and are called optic vesicles. The optic vesicles grow laterally. As they come closer to the layer of surface ectoderm, the optic vesicles stimulate the overlying ectoderm to differentiate into a specialized mass of cells called the lens placode (which will become the lens) by secreting the growth factor BMP4. The ability of the surface ectoderm to respond to BMP4 depends on the expression of the PAX6 gene by the ectoderm.[5]
The Sonic Hedgehog Factor
The development of the eyes involves a delicate interplay between various genes and transcription factors, the most important of which might be sonic hedgehog factor.
Even before neurulation occurs, there already exists the designation of a single eye field in the neural plate. This eye field area divides into two after development of the neural tube. This division can only occur under the influence of sonic hedgehog, which sets off the process that stimulates the growth of two separate eyes and orbital cavities.[6] It is thought that sonic hedgehog expressed from the prechordal plate causes down-regulation of the PAX6 gene and activation of the PAX2 gene, which causes the single eye field to divide into two.
Holoprosencephaly and Shh
Defects in the sonic hedgehog protein Shh or its signaling pathway are implicated in holoprosencephaly, since the Shh is necessary for the brain to develop into distinct left and right lobes. Division of the brain is a process that normally occurs between the eighteenth and twenty-eighth day of gestation. Only subject to this division can the visual cortex begin to develop.
When an embryo reaches development with only a single forebrain region (a condition called alobar holoprosencephaly), it will also be likely to develop only a single eye. When embryos reach the appropriate developmental phase with two separate cerebral hemispheres, they will go on to develop two eyes. Failure of the events that Shh sets into place leads to downstream lack of expression of PAX2, and subsequent lack of down-regulation of PAX6. Expression of PAX2 with down-regulation of PAX6 is necessary for the formation of a distinct visual field corresponding to each hemisphere. Hence problems with Shh signaling can lead to failure to develop two eyes.
Genes implicated in synophthalmia
The development of the forebrain and the eye fields is an intricate interplay between a plethora of genes and transcription factors. The fraction of what has been studied and what is understood is been summarized below.[7]
SHH[8], SIX3[9], TGIF1[10][11], ZIC2[12], PTCH1[13], FOXH1[14], NODAL[15], CDON[16], FGF8[17], GLI2[18], FOXG1[19]
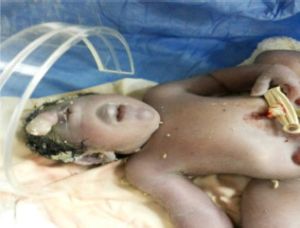
Clinical features
A child with cyclopia presents with microcephaly, since the lobes of the brain and the ventricles are hypoplastic. Perhaps the most striking feature of all, with the exception of a single central eye, is the absence of a nose or the presence of a proboscis above the eye in place of a nose. The single central eye is located in a single central orbit. In some cases it is one single eye (true cyclopia) in others it is partially fused eyes within the single orbit (synophthalmia). The mouth is usually not well formed. In some cases, it is a mound of soft tissue with a cleavage where the mouth would be, with associated micrognathia.
Since cyclopia, (or in a broader sense, alobar holoprosencephaly) is not compatible with life, there are only a few anatomical features, evident on dissection, recorded in literature:[20]
- Complete or near-complete interhemispheric non-separation.
- Absence of falx cerebri
- Absent corpus callosum
- Single midline ventricle
- Absent olfactory bulbs
- Fused deep grey nuclei
- Extra cranial manifestations : polydactyly, omphalocele, renal dysplasia[21]
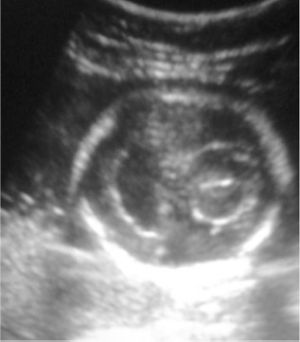
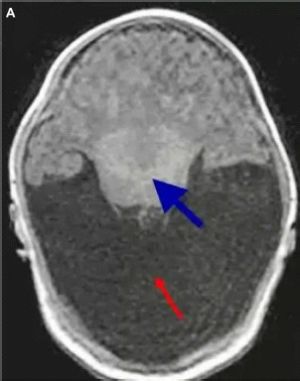
There appears to be a tendency for holoprosencephaly to run in families, but this is only true of the milder varieties of presentation. There is a wide range of presentations that come with varying degrees of holoprosencephaly, and cyclopia is the extreme end of the spectrum.
Screening
Prenatal ultrasound remains the best modality to diagnose holoprosencephaly, and awareness of the range of features in cyclopia (and, varying degrees of holoprosencephaly) can improve the chances of detection and increase accuracy of an early diagnosis. Some ultrasound features that may point to alobar holoprosencephaly are:
- Monoventricle
- Absence of third ventricle
- Absence of interhemispheric fissure
- Absence of corpus callosum / hypoplastic corpus callosum
- Fusion of thalami
- Changes in vasculature of middle and anterior cerebral arteries
- Severe facial deformations
Timely use of ultrasonography in the detection of features of alobar holoprosencephaly in the nuchal translucency (NT) prenatal scan (done at 11 to 136 weeks of gestation), can enable early diagnosis and, if required, termination of pregnancy within the recommended safe limit of gestational age (in accordance with the United States legislation in many states).
Prognosis
The prognosis for cyclopia, which is the extreme presentation of alobar holoprosencephaly, is grave. It is incompatible with life, and it is mostly seen in spontaneous miscarriages and stillbirths. More often than not, death of the fetus usually occurs in utero. In rare cases that a child is born with cylopia, death typically occurs a mere few hours after birth.
The maximum recorded lifespan of a child born with cyclopia is one day.[23]
Cyclopia through the ages
The few recorded cases of cyclopia that have occured in humans remain in a very small proportion as compared to the recorded incidence in other species:
- In 2024, a young mother in Syria was found to have on ultrasound an 11 week fetus with irregularities in the skull bones and severe cerebral malformation. A single orbital cave was also noted. These were confirmed with pathology evaluation following medically induced abortion.[24]
- In 2023, a mother in India was found to have abnormal ultrasound findings concerning for cyclopia and holoprosencephaly at 17 weeks gestation.[25]
- In Indonesia, on September 13th, 2018, a baby with cyclopia was born without a nose and one eye. The baby weighed 2.4 kg and the heart rate was recorded as hundred beats per minute. The child reportedly died seven hours after birth.
- In 2015, a paper published a case of cyclopia a full term baby born at Prince Hashem Military Hospital in Jordan to an 18 year-old otherwise healthy mother. The baby weighed 2900g and had a large omphalocele. [1]
- A paper published in September 2014 reported a child born with microcephaly, cyclopia, cleft palate and no nose. The Apgar score was 7/8/8, and the child died 10 hours after birth.[26]
- In 2006, a baby with cyclopia was born in India. She had marked absence of a nose, and the post-mortem report showed presence of only a single hemisphere. The child passed away one day after her birth.
- In his book, Mutants, On Genetic Variety and the Human Body; Armand Marie Leroi reveals the photograph of a stillborn with cyclopia from 1893 (see photo).
- Going further back into historical archives, a record of a baby with cyclopia born in 1793 was found in Sweden; the child reportedly died after two hours of birth.
- It is not a far leap to assume that the cyclopes of the ancient Greek Mythology were, in fact, inspired from a baby born with cyclopia at a time when congenital abnormalities were feared and thought to be bad omens. According to the Greek mythology, the first literature report is between eighth and seventh century BC in Homer’s Odyssey, where Polyphemus is the one-eyed character who threatens Odysseus. Cyclopia has been represented as a crossover from medicine to art and literature through the centuries.
Additional Resources
- Mutants - On Genetic Variety and the Human body by Armand Marie Leroi.
- Grey's Anatomy - Embryology
- Sharma D, Yadav J, Garg E. Cyclopia syndrome. BMJ Case Rep. 2014;2014:bcr2014203535. Published 2014 Jun 9.
- Three mythic giants for common fetal malformation called "cyclopia": Polyphemus, Tepegöz, and Grendel. Turgut AÇ, Hall WA, Turgut M. Childs Nerv Syst. 2021;37:725–726.
References
- ↑ Jump up to: 1.0 1.1 1.2 1.3 Salama GS, Kaabneh MA, Al-Raqad MK, Al-Abdallah IM, Shakkoury AG, Halaseh RA. Cyclopia: a rare condition with unusual presentation - a case report. Clin Med Insights Pediatr. 2015 Feb 9;9:19-23. CC BY - NC 3.0
- ↑ Orioli IM, Amar E, Bakker MK, et al. Cyclopia: an epidemiologic study in a large dataset from the International Clearinghouse of Birth Defects Surveillance and Research. Am J Med Genet C Semin Med Genet. 2011;157C(4):344-357. doi:10.1002/ajmg.c.30323
- ↑ Orioli IM, Castilla EE. 2010. Epidemiology of holoprosencephaly: Prevalence and risk factors. Am J Med Genet Part C Semin Med Genet 154C:13–21.
- ↑ Summers AD, Reefhuis J, Taliano J, Rasmussen SA. Nongenetic risk factors for holoprosencephaly: An updated review of the epidemiologic literature. Am J Med Genet C Semin Med Genet. 2018 Jun;178(2):151-164. doi: 10.1002/ajmg.c.31614. Epub 2018 May 15. PMID: 29761639; PMCID: PMC6705603.
- ↑ http://education.med.nyu.edu/courses/macrostructure/lectures/lec_images/eye.html
- ↑ https://ghr.nlm.nih.gov/gene/SHH
- ↑ https://rarediseases.org/rare-diseases/holoprosencephaly/
- ↑ Wallis DE, Roessler E, Hehr U, Nanni L, Wiltshire T, Richieri-Costa A, Gillessen-Kaesbach G, Zackai EH, Rommens J, Muenke M. Mutations in the homeodomain of the human SIX3 gene cause holoprosencephaly. Nat Genet. 1999 Jun;22(2):196-8. doi: 10.1038/9718. PMID: 10369266
- ↑ Hehr U, Pineda-Alvarez DE, Uyanik G, et al. Heterozygous mutations in SIX3 and SHH are associated with schizencephaly and further expand the clinical spectrum of holoprosencephaly. Hum Genet. 2010;127(5):555-561. doi:10.1007/s00439-010-0797-4
- ↑ Andoniadou CL, Martinez-Barbera JP. Developmental mechanisms directing early anterior forebrain specification in vertebrates. Cell Mol Life Sci. 2013 Oct;70(20):3739-52. doi: 10.1007/s00018-013-1269-5. Epub 2013 Feb 9. PMID: 23397132; PMCID: PMC3781296.
- ↑ Taniguchi K, Anderson AE, Sutherland AE, Wotton D. Loss of Tgif function causes holoprosencephaly by disrupting the SHH signaling pathway. PLoS Genet. 2012;8(2):e1002524. doi:10.1371/journal.pgen.1002524
- ↑ Herrera E, Brown L, Aruga J, Rachel RA, Dolen G, Mikoshiba K, Brown S, Mason CA. Zic2 patterns binocular vision by specifying the uncrossed retinal projection. Cell. 2003 Sep 5;114(5):545-57. doi: 10.1016/s0092-8674(03)00684-6. Erratum in: Cell. 2003 Oct 3;115(1):125. PMID: 13678579.
- ↑ Chassaing N, Davis EE, McKnight KL, et al. Targeted resequencing identifies PTCH1 as a major contributor to ocular developmental anomalies and extends the SOX2 regulatory network. Genome Res. 2016;26(4):474-485. doi:10.1101/gr.196048.115
- ↑ Silvestri C, Narimatsu M, von Both I, Liu Y, Tan NB, Izzi L, McCaffery P, Wrana JL, Attisano L. Genome-wide identification of Smad/Foxh1 targets reveals a role for Foxh1 in retinoic acid regulation and forebrain development. Dev Cell. 2008 Mar;14(3):411-23. doi: 10.1016/j.devcel.2008.01.004. Erratum in: Dev Cell. 2008 Apr;14(4):633. PMID: 18331719.
- ↑ Bertacchi M, Lupo G, Pandolfini L, et al. Activin/Nodal Signaling Supports Retinal Progenitor Specification in a Narrow Time Window during Pluripotent Stem Cell Neuralization. Stem Cell Reports. 2015;5(4):532-545. doi:10.1016/j.stemcr.2015.08.011
- ↑ Bae GU, Domené S, Roessler E, Schachter K, Kang JS, Muenke M, Krauss RS. Mutations in CDON, encoding a hedgehog receptor, result in holoprosencephaly and defective interactions with other hedgehog receptors. Am J Hum Genet. 2011 Aug 12;89(2):231-40. doi: 10.1016/j.ajhg.2011.07.001. Epub 2011 Jul 28. PMID: 21802063; PMCID: PMC3155179.
- ↑ Vogel-Höpker A, Momose T, Rohrer H, Yasuda K, Ishihara L, Rapaport DH. Multiple functions of fibroblast growth factor-8 (FGF-8) in chick eye development. Mech Dev. 2000 Jun;94(1-2):25-36. doi: 10.1016/s0925-4773(00)00320-8. PMID: 10842056.
- ↑ Furimsky M, Wallace VA. Complementary Gli activity mediates early patterning of the mouse visual system. Dev Dyn. 2006 Mar;235(3):594-605. doi: 10.1002/dvdy.20658. PMID: 16342201.
- ↑ Geng X, Acosta S, Lagutin O, Gil HJ, Oliver G. Six3 dosage mediates the pathogenesis of holoprosencephaly. Development. 2016 Dec 1;143(23):4462-4473. doi: 10.1242/dev.132142. Epub 2016 Oct 21. PMID: 27770010; PMCID: PMC5201039.
- ↑ http://medind.nic.in/ibv/t11/i6/ibvt11i6p457.pdf
- ↑ Salama GS, Kaabneh MA, Al-Raqad MK, Al-Abdallah IM, Shakkoury AG, Halaseh RA. Cyclopia: a rare condition with unusual presentation - a case report. Clin Med Insights Pediatr. 2015;9:19-23. Published 2015 Feb 9. doi:10.4137/CMPed.S21107
- ↑ Gaillard, F., Bickle, I. Alobar holoprosencephaly. Reference article, Radiopaedia.org. (accessed on 10 Dec 2021) https://doi.org/10.53347/rID-10509
- ↑ Leroi, Armand M. Mutants: On Genetic Variety and the Human Body. New York: Viking, 2003. Print.
- ↑ Dakkak T, Mansour M, Jarbouh H, Ktail A. A male fetus with cyclopia was discovered after miscarriage: A rare case report from Syria. Clin Case Rep. 2024 Mar 10;12(3):e8644.
- ↑ Kollu R, Kotamraju S, Uligada S, Varunya M. Fetal Cyclopia, Proboscis, Holoprosencephaly, and Polydactyly: A Case Report With Review of Literature. Cureus. 2023 Feb 2;15(2):e34576.
- ↑ https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4054394/