Boston Type 1 Keratoprosthesis
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
The Boston Keratoprosthesis (KPro) is the most widely used artificial cornea or keratoprosthesis. It is a treatment option for corneal disease not amenable to standard penetrating keratoplasty (PKP) or corneal transplant. Continued advances in design and superior postoperative care have resulted in improved outcomes and an exponential increase in the use of the device in recent years.[1] In 2010, 1188 procedures were performed compared to fewer than 50 in 2002 - to date more than 4500 procedures have been performed worldwide. [2]
History
The concept of an artificial cornea is over 200 years old.[3] The first keratoprosthesis was described in 1789 during the French Revolution by Guillaume Pellier de Quengsy.
[4] The first reported human KPro surgery with a quartz crystal implant was performed by Nussbaum in 1855, although some modern KPro experts note that while Nussbaum may have reported the first human surgery, in fact, Guillaume Pellier de Quengsy’s brother, also an ophthalmologist, may have actually been the first to perform the surgery in a human.
In recent decades multiple synthetic corneas have been pioneered and developed, though only three are principally used in practice: the Boston Keratoprosthesis (Massachusetts Eye & Ear Infirmary, Boston, MA), the AlphaCor (Addition Technology Inc., Des Plaines, IL) [5] and the osteo-odonto keratoprosthesis also known as the ‘OOKP’ (originally described by Strampelli, modified by Falcinelli).[6]
The Boston Keratoprosthesis has evolved from original concept to an established device over the past fifty years under the lifetime leadership of Claes Dohlman, MD, PhD. The device was approved by the FDA for marketing in 1992. An active keratoprosthesis research program continues in Boston, MA and other centers worldwide fostering continued innovation with the device.
Design
The Boston Keratoprosthesis is a collar button design keratoprosthesis. It consists of three components: a front plate with optical stem, a back plate and titanium locking c-ring (See Additional Resources 1 & 2). The most recent design is threadless; during assembly the front and back plates are snapped together with corneal tissue sandwiched in-between, which is then used to suture the device to the eye. It is available in type I and type II formats. (The type II format is reserved for severe end-stage ocular surface disease desiccation, and is similar to the type I device but requires a permanent tarsorrhaphy to be performed through which a small anterior nub of the type II model protrudes). This wiki page will focus on the more commonly used type I device. The type I Boston KPro is available in either a single standard pseudophakic plano power or customized aphakic powers (based on axial length) with adult (8.5 mm diameter) and pediatric (7.0 mm diameter) sized back plates. The device is currently machined from medical grade polymethylmethacrylate (PMMA) by a small, family owned and operated precision machine shop (J.G. Machine Co. , Inc.) in Woburn, Massachusetts .
Recent advances in design have contributed to improved outcomes. First, the addition of holes (at present 16 holes) in the back plate allows diffusion of nutritive aqueous to support donor graft stroma and keratocytes.[7] [8] Second, in 2004, a titanium locking c-ring was added to prevent intraocular disassembly of the device. Third, in 2007, the design was changed from a threaded (screw-type) assembly to a threadless design which simplified assembly and produced less damage to the donor endothelium. [9] The most recent advance in design is the implementation of a titanium back plate which likely improves biocompatibility and retention, and may reduce complications such as retroprosthetic membranes (RPM) and stromal corneal melts.[10] [11] [12]. Just before the COVID pandemic, the FDA approved the newest model of the Boston Type 1 Keratoprosthesis - the Lucia design. This design helps to confer a more natural aesthetic appearance while increasing corneal aqueous contact by changing the shape of the titanium corneal backplate. There is consideration to have availabilities limited to two models - phakic and pseudophakic.
Indications
Although traditional penetrating keratoplasty (PKP) or corneal transplantation is an established treatment for some forms of corneal blindness, some conditions are not amenable to PKP. Indications for the Boston KPro include multiple graft failures, Stevens-Johnson syndrome (SJS)[13] , ocular cicatricial pemphigoid (OCP), other autoimmune diseases[14] , ocular burns (acid and alkali)[15][16] and other conditions with poor prognosis with traditional PKP. Currently, the Boston KPro is considered by some to be a primary treatment option in cases of repeat graft failure[17] and aniridia[18], and, by a smaller percentage of surgeons, to be a primary option in cases of herpetic keratitis[19] and pediatric corneal opacities[20] . Pediatric KPro use in particular is still a controversial topic that is widely debated among corneal specialists because of the high level of care needed postoperatively and the possibly devastating complications of the procedure.
Surgical Procedure
Prosthokeratoplasty is the term for a procedure in which a damaged cornea is replaced with an artificial cornea. During implantation of the Boston KPro, the device is assembled with a donor corneal graft positioned between the front and back plate, that is then sutured into place in a similar fashion to PKP. The surgical procedure may be carried out by any surgeon familiar with PKP, and without the worry of astigmatism due to the rigidity of the assembled device. The current recommendation is removal of the crystalline lens at the time of KPro surgery given the inevitable development of cataract as a result of several factors, including long-term postoperative topical steroid use, surgical trauma and other factors[21] . The primary keratoprosthesis surgery is often combined with other procedures including iridoplasty, glaucoma filtration devices, IOL and lens capsule removal and core vitrectomy.
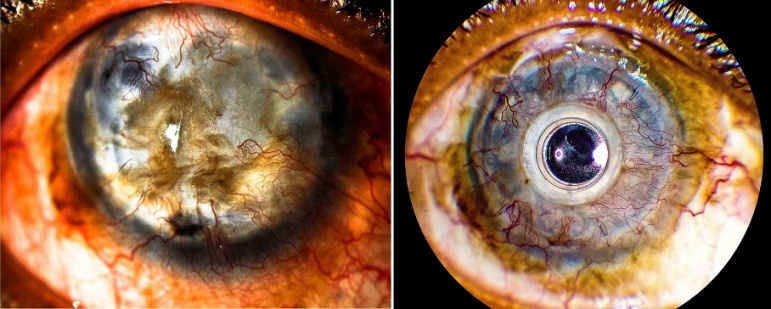
Preoperative & Postoperative Photo
On the left, preoperative photo of an eye with keratoconus and hand motion vision after multiple failed grafts. This eye had a history of multiple filtration surgeries for advanced glaucoma. On the right, seven month postoperative photo after implantation of Boston type 1 KPro. Preliminary vision was 20/30 despite an early RPM.
Prognostic Categories
The Boston KPro can provide visual rehabilitation for severely diseased corneas at high risk for failure with PKP. However, preoperative diagnosis influences clinical outcome after KPro surgery.[22] Many studies have shown the incidence of repair procedures and worse final vision outcomes were higher in groups with autoimmune conditions (SJS, OCP). The difference in outcomes appears to be related to the degree and cumulative past period of inflammation. Overall most favorable outcomes are achieved in non-cicatrizing conditions, followed by ocular burns and OCP with the worst outcomes in SJS patients.
Postoperative management
Closely monitored, long-term follow-up with an ophthalmologist familiar with the KPro is a necessity with the device. Due to the substantial risks of glaucoma and endophthalmitis, it may be best to manage these patients in a team setting consisting of a corneal specialist, glaucoma specialist, and retinal specialist. It must be stressed that KPro eyes require life-long follow-up entailing the commitment of the both the patient and ophthalmologist. The rigorous follow-up should guide patient selection. Furthermore, patient compliance with postoperative medication is of paramount importance. KPro surgery in a historically non-compliant patient should proceed with extreme caution.
Due to incomplete biointegration of hardware in the eye there is a significant risk of infectious endophthalmitis[23] , a devastating complication which often results in a substantial loss of vision. The incidence of IE has been significantly reduced by life-time daily topical antibiotic prophylaxis.[24] The optimal postoperative antibiotic prophylaxis is not universally agreed upon. Typical regimes include a topical fourth generation fluoroquinolone (moxifloxacin or gatifloxacin) with or without topical vancomycin. The increase in incidence of gram-positive IE cases in one study was the basis for the addition of topical vancomycin (preserved with 0.005% benzalkonium chloride which allows storage at room temperature for up to 60 days – this lowered cost and improved patient compliance) to the prophylaxis regime in some patients. Most surgeons recommend a topical fluoroquinolone and topical vancomycin in the initial postoperative period of all KPro eyes and then continuing dual agent coverage in autoimmune (a group at higher risk for IE) or monocular patients. In lower risk groups, a single agent topical fluoroquinolone or polymyxin B/trimethoprim 2-3 times daily can provide effective long-term antibiotic prophylaxis. Some KPro surgeons recommend antibiotic cycling to prevent microbial resistance, although there are no published studies to support this. Regardless of regime employed, the incidence of this devastating complication is reduced with permanent topical antibiotic prophylaxis and patients should be educated on the important of compliance.
Another postoperative management intervention is the indefinite placement of a bandage contact lens (BCL).[25] Placement of a BCL is needed to maintain adequate ocular surface hydration and prevent stromal melt, dellen formation and necrosis. The BCL has many other added benefits including improving patient comfort and protecting from possible exposed sutures. The benefit of the BCL is multifactorial and can include other therapeutic, refractive and cosmetic roles. Ocular surface exposure can lead to KPro melt and failure and sometimes a tarshorraphy is necessary in addition to the BCL. The downside to BCL use is the increased risk of infection associated with contact lens use, especially when used on a chronic, extended wear basis.
Life-long topical steroids such as prednisolone acetate is necessary in all KPro eyes to prevent inflammation and has been successfully utilized since inception of the device.[8][26] Since steroids can cause IOP elevation, reduce host defenses and inhibit wound healing, KPro patients require close follow-up and monitoring for these and other potential complications.
Complications
The three most commonly reported postoperative complications are retroprosthetic membrane (RPM), elevated intraocular pressure/glaucoma and infectious endophthalmitis (IE) which will be discussed here.[27] [28] [29] [30] Other less frequent complications include sterile vitritis, stromal melt, retinal detachment and vitreous hemorrhage.
The incidence of RPM in clinical series is reported to be between 25 and 65%. It is believed inflammation is the most important factor for RPM formation [11]. Most cases are successfully treated with simple, single session YAG membranotomy. Typically surgical membranectomy is reserved for cases refractory to YAG laser treatment. The YAG laser should be used with caution at energy greater than 3.0 mJ as it can crack or pockmark the KPro optic and appropriate technique and laser offsets should be used. [31] To avoid surgical membranectomy, RPM should be treated before it progresses, thickens and becomes progressively more vascularized rendering it less suitable to YAG membranotomy. Despite best efforts at RPM monitoring and treatment with YAG laser, some patients will ultimately still progress and require more invasive surgical intervention.
With the significant reduction in IE with current antibiotic regimens, glaucoma is now the most significant threat to vision in KPro patients. The reported incidence of glaucoma preoperatively in KPro eyes is 60-76% and postoperative high IOP has been shown in 15-38% of KPro eyes. Gradual closure of the anterior chamber angle is suspected as the etiology.[26] Monitoring IOP is challenging as traditional tonometry cannot be used with the device in place and measurements rely on digital palpation of the sclera. This complication of KPro surgery is optimally managed in close consultation with an experienced glaucoma specialist and often requires filtration surgery with aqueous shunts and aggressive topical IOP-lowering agents.[26][32] [33]
Infectious endophthalmitis is a catastrophic complication often resulting in loss of vision. The most comprehensive series reports the overall incidence of IE as 2.7% per patient year[24]--in effect one of the highest endophthalmitis rates of any ophthalmic surgical procedure currently performed. For perspective, this is 67.5x's higher than the rate for cataract surgery, and 13.5x's higher than the rate for glacuoma filtering surgery. Clinically this complication presents as a sudden onset of ocular pain, scleral injection, and intraocular inflammation manifested by anterior and/or vitreous turbidity (often with hypopyon) and decreased vision. This is in contrast to sterile vitritis which also presents with a decrease in vision. On exam, flocculent vitritis is seen but typically without the pain, tenderness or conjunctival injection seen with IE.[26][34] Final vision after an episode of IE is closely correlated with the causative agent - eyes infected with Streptococci or Staph. Aureus (especially methicillin-resistant strains (MRSA)) result in poorer vision when compared to Staph. epidermis or Strep. viridians infections. If IE occurs, immediate evaluation and treatment is required. Aqueous and/or vitreous samples should be examined and cultured for bacteria and fungi. Intravitreal injection of vancomycin, amikacin and dexamethasone is recommended and pars plana vitrectomy is often necessary. Hospitalization should be considered in order to guarantee compliance with aggressive topical treatment, post-surgical care and possible IV antibiotics.[26]
Outcomes
Four major clinical studies, summarized below, have reported outcomes with the type I Boston KPro. Our series at Weill Cornell Medical College, currently in press, is also summarized.
- Results from the Multicenter Boston Type 1 Keratoprosthesis Study: This is the largest study to date and involved multiple surgeons at various medical centers. This seminal study involved 141 Boston type I keratoprosthesis procedures from 17 surgical sites by 39 different surgeons. At an average follow-up of 8.5 months, retention rate of the device was 95%, 57% had BCVA ≥ 20/200, 19% had BCVA ≥ 20/40. Postoperative complications included RPM in 25%, high IOP in 15%, and sterile vitritis in 5% of eyes. Importantly, no cases of infectious endophthalmitis were reported in this large series.[35]
- The Boston Type 1 Keratoprosthesis: Improving Outcomes and Expanding Indications: This is the largest single surgeon (Anthony Aldave, MD) series which included 57 modern type I Boston KPro procedures. At an average follow-up of 17 months, retention rate of the device was 84%, 75% had BCVA ≥ 20/200, 43% had BCVA ≥ 20/50 (BCVA for N=34, eyes with at least 1 year follow-up). Postoperative complications included RPM in 44%, high IOP in 18%, and sterile vitritis in 10% of eyes. No cases of infectious endophthalmitis were reported in this series.[29] This study is notable for a subset of eight eyes in which no prior corneal surgery had been done. In this subset the retention rate was 100% and two of these patients had SJS suggesting that better outcomes might be achieved if KPro surgery is an early surgical procedure rather than a measure of last resort.
- Boston Type 1 Keratoprosthesis: The University of California Davis experience: A single center study from UC Davis of 30 Boston type I KPro procedures. At an average follow-up of 19 months, retention rate of the device was 83%, 77% had BCVA ≥ 20/200, 23% had BCVA ≥ 20/40. Postoperative complications included RPM in 43%, high IOP in 27%, and sterile vitritis in 3% of eyes. The rate of infectious endophthalmitis in this study was 10%.[28]
- Boston Keratoprosthesis Outcomes and Complications: A retrospective study of 36 Boston Type I KPro procedures from Wills Eye Institute. At an average follow-up of 16 months, retention rate of the device was 100%, 83% had BCVA ≥ 20/200, 44% had BCVA ≥ 20/50. Postoperative complications included RPM in 65% and high IOP in 38%. Infectious endophthalmitis complicated 11% of eyes during the postoperative period.[27]
- Our in-press series from Weill Cornell Medical Center includes 20 Boston type I KPro procedures by 4 different surgeons. At an average follow-up of 13.5 months, retention rate of the device was 82%, 53% had BCVA ≥ 20/200, 35% had BCVA ≥ 20/80. Postoperative complications included RPM in 53%, high IOP in 35%, sterile vitritis complicated the postoperative course of 5.8% of eyes. The rate of infectious endophthalmitis in this study was 5.8%.
A pooled analysis of the above five clinical series yielded 278 type I Boston KPro procedures performed in 263 eyes of 256 patients. Average follow-up (weighted average) in this cohort was 12.6 months with an overall initial KPro retention rate of 91%. 67% had BCVA ≥ 20/200 (for N=145, given BCVA data available for eyes with at least 1 year follow-up). Postoperative complications in this group included RPM in 39.1%, elevated IOP in 22%, stromal melt in 7.6%, vitreous hemorrhage in 5.3% and retinal detachment in 4.6%; sterile vitritis complicated the postoperative course of 5.3% of eyes. Among all eyes, the post-operative rate of infectious endophthalmitis was 3%.
International Use
The WHO estimates that approximately 8 million people worldwide suffer from corneal blindness with the majority living in developing countries. Pilot projects have been conducted in multiple countries including Sudan, Ethiopia, India and China with preliminary but promising results.[36] Of note, given the lack of corneal tissue banks in developing countries, use of a patient’s own ipsilateral autologous cornea for assembly of the KPro has shown initial success.[37] Future studies with longer follow-up are needed to determine if the Boston KPro is a safe and economical device for corneal disease in developing countries.
Cost
As of January 1, 2010 the cost of the Boston KPro rose to $5,000 (increased from $3,000 previously).[12] The increase is attributed to larger research expenditures and rising regulatory costs. Nevertheless, the device is priced on an international sliding scale and is provided at a substantial discount to poorer nations.
Reimbursement by Medicare, Medicaid, and other insurers for keratoprosthesis surgery has largely been successful. However, certain conditions must often be met prior to surgery. For instance, Blue Cross will only reimburse for the surgery after two failed traditional corneal transplants, but this has been amended in certain states to include reimbursement for KPro as a primary surgery. Thorough dictation which clearly states the indications for keratoprosthesis surgery is often necessary and can aid in collecting payor reimbursement. Typically reimbursement can be collected for keratoprosthesis placement ($1,552 in Massachusetts) along with 50% payment for the additional PKP performed at the time of KPro placement (aphakic CPT 65750 $1280@50% = $640, pseudophakic CPT 65755 $1276@50%=$638). Additional procedures at the time of KPro surgery including aspiration of cataract (CPT 66840), cataract with IOL (CPT 66984) and secondary IOL (CPT 66985) among others have also been reimbursed at 50% of allowable. Facility payments can also be billed separately and the specific reimbursement amount is dependent on insurance carrier. Billing codes for facilities include CPT 65770 (KPro surgery), HCPCS code C1818 (KPro Device), HCPCS code V2785 (corneal tissue).
A recent study on the cost-effectiveness of the Boston KPro utilizing quality-adjusted life years (QALY) as a measure of comparative effectiveness found the Boston Keratoprosthesis provided a QALY gain of 20.3% to the average patient. The average cost-effectiveness of KPro surgery was $16,140 per QALY which is on par with the benefit received from penetrating keratoplasty estimated to be approximately $12,194 per QALY.[38]
Lucia Keratoprosthesis
The latest iteration of the Boston KPro aims to enhance affordability and cosmetic appearance of the Boston KPro. For this purpose, the latest design termed "Lucia" keratoprosthesis recieved FDA approval in 2019. [39] Modifications in the backplate include improved fabrication time and reduced costs by photoetching and bending, instead of machining the titanium on a lathe. Also, the only available size comes in a 7.8 mm backplate diameter, standardazing sizes and reducing inventory holding costs.
A more physiological rather than "robotic" appearance is sought after KPro implantation. For this purpose, the backplate of the Lucia KPro features 16 petaloid radial slits, simulating a normal iris. In addition, the titanium comes anodized, which is able to provide a brown or blue tonality, further improving cosmetic results. [39] Clinical outcomes with this design and a cost-effectiveness analysis are warranted.
Future Directions
Research actively continues on the Boston Keratoprosthesis to improve design and outcomes and to expand indications for its use. KPro patients are prone to develop inflammation, RPM and cellular debris within and around the PMMA back plate, and studies are investigating alternative keratoprosthesis materials including titanium.[11][40] [41] The optimal management of glaucoma and projects aimed at accurately assessing IOP in KPro eyes utilizing intraocular pressure transducers with telemetry capabilities are underway.[42] Given the permanent need for a BCL, investigators continue to explore drug-eluting contact lenses, which could circumvent the need for patient initiated delivery of topical medications and is likely to improve outcomes given enhanced, reliable delivery of drugs.[40][43] Researchers continue to challenge the dogma that KPro surgery should not be done in patients with good vision in a fellow eye, and are investigating restoring useful binocular vision in patients with corneal disease.[44] Investigators are also pursuing improving clinical outcomes with the Boston KPro in autoimmune conditions and chemical burns which have traditionally been plagued with poorer outcomes. Recently, the use of soft skirt materials has also been of great interest between both biomedical engineering and ophthalmic care as an approach to increase bio-integration of the synthetic cornea without the need for graft donation.[45] Fully artificial keratoprostheses would provide opportunity for KPro candidates whose circumstances do not allow for viable access to donor graft tissue, a large problem due to the global shortage of donor corneas.[46] Retention rates of fully artificial cornea prosthetics have not been met with much success in the past and requiring eventual explantation, but newer models of artificial cornea devices, such as the KeraKlear Artificial, Gore Synthetic, and CorNeat KPro, have shown recent promise in animal models.[45][47] However, the significant bio-integrative advantages and clinical success of the Boston Type and Osteo-Odonto KPro models have continued to popularize their use and research.[48]The Boston KPro has made remarkable strides over the past decade and its continued success is promising.
Additional Resources
- Digital Journal of Ophthalmology (DJO). Modern design of Boston Keratoprosthesis. http://www.djo.harvard.edu/files/6425_1055.jpg Boston, MA. Accessed April, 4, 2019.
- Digital Journal of Ophthalmology (DJO). Illustration of assembled device. http://www.djo.harvard.edu/files/6426_1055.jpg Boston, MA. Accessed April, 4, 2019.
- KPro Study Group
- Additional video of surgical procedure
References
- ↑ Klufas, M.A. and C.E. Starr, The Boston Keratoprosthesis : An update on recent advances. Cataract and Refractive Surgery Today September 2009. 9(9).
- ↑ Klufas, M.A. and K.A. Colby, The Boston keratoprosthesis. Int Ophthalmol Clin, 2010. 50(3): p. 161-75.
- ↑ Chirila, T.V. and C.R. Hicks, The origins of the artificial cornea: Pellier de Quengsy and his contribution to the modern concept of keratoprosthesis. Gesnerus, 1999. 56(1-2): p. 96-106.
- ↑ Pellier de Quengsy, G., Précis au cours d'operations sur la chirurgie des yeux. 1789, Paris: Didot.
- ↑ Hicks, C.R., et al., Outcomes of implantation of an artificial cornea, AlphaCor: effects of prior ocular herpes simplex infection. Cornea, 2002. 21(7): p. 685-90.
- ↑ Liu, C., et al., The osteo-odonto-keratoprosthesis (OOKP). Semin Ophthalmol, 2005. 20(2): p. 113-28.
- ↑ Khan, B.F., et al., Advances in Boston keratoprosthesis: enhancing retention and prevention of infection and inflammation. Int Ophthalmol Clin, 2007. 47(2): p. 61-71.
- ↑ Jump up to: 8.0 8.1 Harissi-Dagher, M., et al., Importance of nutrition to corneal grafts when used as a carrier of the Boston Keratoprosthesis. Cornea, 2007. 26(5): p. 564-8.
- ↑ Dohlman, C. and M. Harissi-Dagher, The Boston Keratoprosthesis: A New Threadless Design. Digital Journal of Opthalmology, 2007. 13(3).
- ↑ Ament, J.D., et al., The Boston Keratoprosthesis: Comparing Corneal Epithelial Cell Compatibility with Titanium and PMMA. Cornea, 2009.
- ↑ Jump up to: 11.0 11.1 11.2 Dohlman, C.H., et al., Titanium vs. PMMA Backplates for Boston Keratoprosthesis: Incidence of Retroprosthetic Membrane. ARVO, 2009. Program Number 1505: p. Poster A415.
- ↑ Jump up to: 12.0 12.1 Newsletter VII, in Boston Keratoprosthesis Update, R. Walcott-Harris and C. Dohlman, Editors. 2010.
- ↑ Sayegh, R.R., et al., The Boston keratoprosthesis in Stevens-Johnson syndrome. Am J Ophthalmol, 2008. 145(3): p. 438-44.
- ↑ Ciralsky, J., et al., Keratoprosthesis in autoimmune disease. Ocul Immunol Inflamm, 2010. 18(4): p. 275-80.
- ↑ Tuft, S.J. and A.J. Shortt, Surgical rehabilitation following severe ocular burns. Eye, 2009.
- ↑ Harissi-Dagher, M. and C.H. Dohlman, The Boston Keratoprosthesis in severe ocular trauma. Can J Ophthalmol, 2008. 43(2): p. 165-9.
- ↑ Ma, J.J., J.M. Graney, and C.H. Dohlman, Repeat penetrating keratoplasty versus the Boston keratoprosthesis in graft failure. Int Ophthalmol Clin, 2005. 45(4): p. 49-59.
- ↑ Lee, H., R. Khan, and M. O'Keefe, Aniridia: current pathology and management. Acta Ophthalmol, 2008. 86(7): p. 708-15.
- ↑ Khan, B.F., et al., The Boston keratoprosthesis in herpetic keratitis. Arch Ophthalmol, 2007. 125(6): p. 745-9.
- ↑ Aquavella, J.V., et al., Pediatric keratoprosthesis. Ophthalmology, 2007. 114(5): p. 989-94.
- ↑ Harissi-Dagher, M. and K.A. Colby, Cataract extraction after implantation of a type I Boston keratoprosthesis. Cornea, 2008. 27(2): p. 220-2.
- ↑ Yaghouti, F., et al., Keratoprosthesis: preoperative prognostic categories. Cornea, 2001. 20(1): p. 19-23.
- ↑ Nouri, M., et al., Endophthalmitis after keratoprosthesis: incidence, bacterial causes, and risk factors. Arch Ophthalmol, 2001. 119(4): p. 484-9.
- ↑ Jump up to: 24.0 24.1 Durand, M.L. and C.H. Dohlman, Successful prevention of bacterial endophthalmitis in eyes with the Boston keratoprosthesis. Cornea, 2009. 28(8): p. 896-901.
- ↑ Harissi-Dagher, M., J. Beyer, and C.H. Dohlman, The role of soft contact lenses as an adjunct to the Boston keratoprosthesis. Int Ophthalmol Clin, 2008. 48(2): p. 43-51.
- ↑ Jump up to: 26.0 26.1 26.2 26.3 26.4 Dohlman, C.H., et al., Introduction to the use of the Boston keratoprosthesis. Expert Review of Ophthalmology, 2006. 1(1): p. 41-48.
- ↑ Jump up to: 27.0 27.1 Chew, H.F., et al., Boston keratoprosthesis outcomes and complications. Cornea, 2009. 28(9): p. 989-96.
- ↑ Jump up to: 28.0 28.1 Bradley, J.C., et al., Boston type 1 keratoprosthesis: the university of california davis experience. Cornea, 2009. 28(3): p. 321-7.
- ↑ Jump up to: 29.0 29.1 Aldave, A.J., et al., The Boston type I keratoprosthesis: improving outcomes and expanding indications. Ophthalmology, 2009. 116(4): p. 640-51.
- ↑ Akpek, E.K., et al., Outcomes of Boston keratoprosthesis in aniridia: a retrospective multicenter study. Am J Ophthalmol, 2007. 144(2): p. 227-231.
- ↑ Chak, G. and J.V. Aquavella, A safe Nd:YAG retroprosthetic membrane removal technique for keratoprosthesis. Cornea, 2010. 29(10): p. 1169-72.
- ↑ Dohlman, C.H., et al., Shunts to divert aqueous humor to distant epithelialized cavities after keratoprosthesis surgery. J Glaucoma, 2010. 19(2): p. 111-5.
- ↑ Rivier, D., et al., Glaucoma and keratoprosthesis surgery: role of adjunctive cyclophotocoagulation. J Glaucoma, 2009. 18(4): p. 321-4.
- ↑ Nouri, M., M.L. Durand, and C.H. Dohlman, Sudden reversible vitritis after keratoprosthesis: an immune phenomenon? Cornea, 2005. 24(8): p. 915-9.
- ↑ Zerbe, B.L., M.W. Belin, and J.B. Ciolino, Results from the multicenter Boston Type 1 Keratoprosthesis Study. Ophthalmology, 2006. 113(10): p. 1779 e1-7.
- ↑ Ament, J.D., et al., Global corneal blindness and the Boston keratoprosthesis type I. Am J Ophthalmol, 2010. 149(4): p. 537-9.
- ↑ Ament, J.D., et al., Role for ipsilateral autologous corneas as a carrier for the Boston keratoprosthesis: the Africa experience. Arch Ophthalmol, 2010. 128(6): p. 795-7.
- ↑ Ament, J.D., et al., Cost-effectiveness of the Boston keratoprosthesis. Am J Ophthalmol, 2010. 149(2): p. 221-228 e2.
- ↑ Jump up to: 39.0 39.1 Bakshi SK, Paschalis EI, Graney J, Chodosh J. Lucia and Beyond: Development of an Affordable Keratoprosthesis. Cornea 2019;38:492–497.
- ↑ Jump up to: 40.0 40.1 Ciolino, J.B. and C.H. Dohlman, Biologic keratoprosthesis materials. Int Ophthalmol Clin, 2009. 49(1): p. 1-9.
- ↑ Ament, J.D., et al., The Boston Keratoprosthesis: comparing corneal epithelial cell compatibility with titanium and PMMA. Cornea, 2009. 28(7): p. 808-11.
- ↑ Todani, A., et al., Measurement of IOP With Intraocular Pressure Transducer. ARVO, 2009. Program Number 5220: p. Presentation Abstract.
- ↑ Ciolino, J.B., et al., A drug-eluting contact lens. Invest Ophthalmol Vis Sci, 2009. 50(7): p. 3346-52.
- ↑ Pineles, S.L., et al., Binocular Visual Function in Patients With Boston Type I Keratoprostheses. Cornea, 2010.
- ↑ Jump up to: 45.0 45.1 Fu L, Hollick EJ. Artificial Cornea Transplantation. In: StatPearls. Treasure Island (FL): StatPearls Publishing; April 20, 2023.
- ↑ Kinoshita S. The Future of Corneal Transplantation. Ophthalmology Management. 2024;28(June 2024):20-21
- ↑ Promising New Artificial Corneas. American Academy of Ophthalmology. Published November 15, 2021. https://www.aao.org/eyenet/academy-live/detail/promising-new-artificial-corneas.
- ↑ Musa M, Zeppieri M, Enaholo ES, Chukwuyem E, Salati C. An Overview of Corneal Transplantation in the Past Decade. Clinics and Practice. 2023; 13(1):264-279. https://doi.org/10.3390/clinpract13010024.