Anterior Segment Trauma: Evaluation, Considerations and Initial Management
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
Corneal Trauma
Introduction
The anterior segment of the eye is constantly exposed to minor trauma throughout the normal day. The precorneal tear film, corneal epithelium, and conjunctival epithelium provide protection against this continued mild trauma; however, significant trauma can occur which requires ophthalmic examination and treatment. This article discusses anterior segment trauma, how to evaluate it, and initial considerations in its management.
Corneal Burns
Thermal
Thermal injuries result from heat damage to ocular tissues. The etiology and mechanism of action of the thermal injury can provide important information that will help to determine the extent of the tissue damage. Thermal injuries may result from contact with hot objects, such as a curling iron, hot liquids, and fire. It is vital to remember that ocular injuries from fires may occur in association with burns in other regions of the body. This type of injury is frequently unilateral. Studies have determined that long-term sequelae were rare and seen in only 3% of patients with corneal burns.[1] [2] Although uncommon, thermal injuries in children should always be evaluated with the possibility that they were the result of child abuse, especially in the setting of multiple past injuries.
Common injuries in the home, such as with curling irons, are usually mild with resolution of signs and symptoms within 1 to 2 week after onset with proper wound care, topical antibiotics, cycloplegia, and pressure patching.[3]
As in chemical burns, limbal involvement is an important prognostic factor in determining the long-term outcome. The treatment for severe burns from molten metal and fireworks may involve other ocular structures, which require medical or surgical repair, including limbal stem cell transplantation.[4] Experimental adjunctive therapies may be beneficial. Such therapies include delivering oxygen to the burned ocular surface using a face mask for 1 hour a day, which Sharifipour et al. found to reduce limbal ischemia, accelerate epithelialization, and decreasing corneal vascularization.[5] For those patients with the loss of eyelid tissue, a study demonstrated that the use of a gas permeable scleral contact lens may be helpful to decrease corneal exposure; however, repair of the lids is critical for the long term health of the ocular surface.[6]
Ultraviolet Light
The short, high energy wavelength (40-400 nanometer energy) properties of UV light can damage ocular tissue, especially the cornea, conjunctiva, or lens. Corneal/conjunctival UV light injuries commonly result from exposure to sunlight, tanning lamps, and welding arcs, and are usually bilateral. Minor UV light injuries result in punctate keratitis and conjunctival chemosis, which usually occurs 3–12 h after exposure. Patients typically experience pain, tearing, and blepharospasm, but symptoms are usually self-limited and resolve after re-epithelialization. [7] Patients may be treated symptomatically with lubricants. Topical antibiotics may be used if the keratitis is significant but antibiotics that are less toxic to the corneal epithelium should be selected in order to avoid delayed healing. Although topical anesthetics provide immediate pain relief, these are not recommended for use outside the examination, due to the high abuse potential, which is associated with severe corneal complications. Chronic exposure to UV can cause premature cataract or acceleration of cataract formation.
Chemical Injury
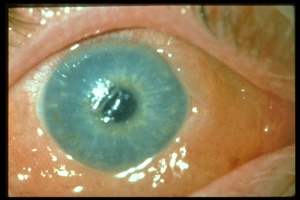
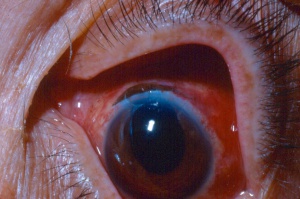
Chemical injury is the most common cause of a corneal burn. Chemical injuries to the eyes occur in the home, industrial setting, farm and other sites. Chemical injuries from assaults represent approximately 11% of chemical injuries and tend to result in severe injuries.[7] In general, immediate irrigation with sterile saline solution (or tap water, if a sterile saline solution is not available) should be started as soon as possible. Then, a thorough history and physical examination can take place. The chemical agent should be identified, if possible. The OSHA chemical identification standard data (CISD or SDS) will identify the chemical(s), toxicity, and possible treatment, if it is available. Otherwise, the poison control center may be able to provide this information. Both eyes should be thoroughly examined since 42% of injuries are bilateral.[8] Acid and alkali agents can be assessed by pH testing of the agent or the affected tissue. Alkali injuries tend to be more severe than acid injuries because alkali agents are hydrophilic and lipophilic, causing them to rapidly damage cell walls and penetrate ocular tissues deeper. Chemical solvents, such as acetone, which has a neutral pH, have been reported to cause corneal stem cell damage after being used to remove cosmetic eye lash extensions.[8]
Acid Injuries
The major settings in which acid injuries occur are: laboratories, industry, and the home; while the most common acids involved in ocular injuries, in order of prevalence, are sulfuric, nitric, hydrochloric, and oxalic acid.[8] Automobile batteries release sulfuric acid when they explode. Hydrofluoric acid tends to cause more severe injuries due to its increased tissue penetration and the added effect of its fluoride ions.[9] The penetration of acids is reduced by ocular tissue as the acid causes protein precipitation and denaturation, which acts as chemical buffer.[10] If the limbal stem cells are damaged, the long-term prognosis is poor and is related to the number of clock hours of the limbus affected and the degree of total stem cells lost. Direct acid related tissue damage, secondary inflammation, and fibrosis can result in secondary glaucoma and cataract formation.[11]
Alkaline Injuries
Alkali injuries are generally more severe than acid injuries because of their lipophilic effects and their ability to penetrate the cornea through saponification of cell membrane components which results in cellular destruction.[12] Alkali is a common cause of ocular chemical trauma. Sodium hydroxide, calcium hydroxide, and ammonium hydroxide are a few of the most common alkali agents involved in ocular injuries.[7] Common household items containing alkali agents are bathroom cleaners, plaster, lye, lime, cement, and ammonia.[8]
The Initial Treatment of Chemical Injuries
The OSHA chemical identification standard data (CISD or SDS) will identify the chemical(s), toxicity, and possible treatment(s), if it is available. Both eyes should be thoroughly examined. Chemical burns should generally be irrigated with sterile saline solution or Ringer's lactate. Treatment should begin immediately and start with irrigation of the ocular tissues to dilute, neutralize and remove the acid or base, which reduces the extent of tissue damage.[13]
Irrigating solutions differ in their ability to effectively normalize the pH. Because water is hypotonic it diffuses through the de-epithelialized cornea and is not as effective as other irrigating agents with higher osmolarity. [14] However, water is useful when other irrigating solutions are not available. If available, solutions with buffering capacity are preferred; Previn, Diphoterine, or Cederroth Eye Wash solution are superior in balancing the pH, based on testing with experimental models.[15] Irrigation should last at least 15 minutes with at least 1000 ml of irrigation solution with confirmation of normalization of the pH with litmus paper strips and a secondary pH test 10 minutes later to confirm neutralization of the acid or base. A Morgan lens, or similar device, can be helpful to instill the irrigating solution. Instilling a topical anesthetic can provide additional comfort before irrigation and examination of the eye. Care should be taken to irrigate the fornices to ensure that trapped chemicals are not missed. [8]
Magnesium hydroxide, which is present in sparklers and other fireworks can cause both a thermal and alkali injury. Before irrigation, a cotton tip applicator can be initially used to brush the dry magnesium out of the eye. Similarly, any superficial particulate matter from other chemical injuries should be removed with forceps or a cotton tip applicator. After irrigation, a thorough ocular examination can be performed including lid eversion, to detect any retained debris. The use of ointments is not recommended immediately after a chemical injury since this could result in potentially trapping retained chemicals and toxins.[8]
Long term clinical care and possible surgery should be discussed with the patient. Due to the loss of vision, the injured person may need physical guidance.[14] There are many established and new treatment that can be used to enhance healing and reduce long term complications of the chemical injury.
Corneal Abrasion
Corneal abrasions are one of the most common types of injuries to the anterior segment. One study documented that among all emergency room visits in patients with ocular complaints, approximately 24.3% presented with corneal abrasions.[16] A corneal abrasion occurs when the corneal epithelium is physically removed from the corneal surface. The most common etiologies of corneal abrasion involve the following causes: fingernails, sport related trauma, make-up brushes, and airbags. Children cause many fingernail injuries to the cornea, as patients are often parents who become injured while holding a small child.[17] Automobile airbags can cause corneal abrasions and are frequently associated with blunt force trauma as well as alkali burns.[18] Corneal abrasions can also occur in the hospital setting. These incidents may occur during surgery, when the patient is under anesthesia, and are not recognized until the patient awakens; or they may occur in an admitted unconscious patient.[19]
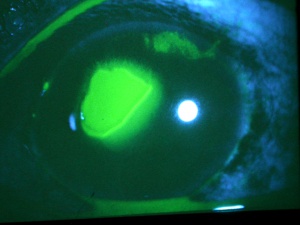
Corneal abrasions result in acute pain, tearing, blurred vision, photophobia, and a foreign body sensation. A thorough history is always important since the mechanism of trauma can provide much useful information. If plant or other organic matter was involved, the risk of bacterial and especially fungal contamination must be considered in order to guide the choice of prophylactic medications. Injuries that are associated with blunt trauma should alert the physician to the possibility of additional intraocular and extraocular injuries. A complete ophthalmic examination should be completed to rule out other injuries and a Seidel test should be performed if there is any suspicion of anterior chamber penetration. Corneal abrasions and lacerations from fingernail injuries are prone to result in recurrent erosions due to disruption of the corneal epithelium’s basement membrane. Topical anesthetic eye drops, such as tetracaine or proparacaine, will reduce the patient’s pain and tearing, and usually make the ocular examination more comfortable for the patient and thus allow the examiner to perform a more complete examination. Anesthetic drops should not be used beyond the exam room as they can lead to devastating complications, such as corneal melts. Abrasions may be visible to the examiner without magnification. Typically, the corneal light reflex is disrupted and the area of the abrasion may appear dull or hazy. Fluorescein dye adheres to the basement membrane and corneal stroma, where the corneal epithelium has been lost. The dye fluoresces bright green with a cobalt blue light source, which elucidates the extent of the injury.
Treatment should be initiated to protect against infection and promote healing with antibiotic drops or ointments and ocular lubricants. Pseudomonas antibiotic coverage should be considered in contact lens wearers. If pain is severe, NSAIDS or Tylenol may be used, A bandage contact lens or pressure patch can placed for select patients.
Corneal Foreign Bodies
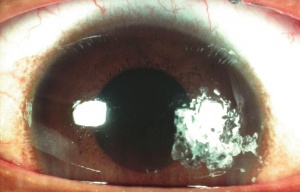
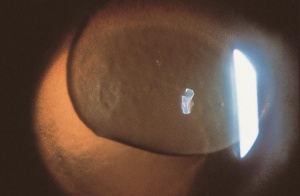
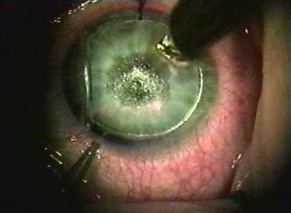
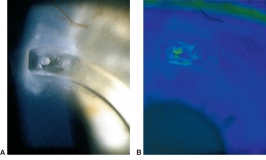
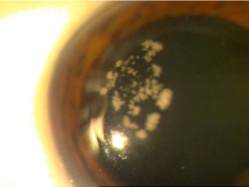
Corneal foreign bodies are the result of a material becoming imbedded in corneal tissue. Corneal foreign bodies frequently occur in the workplace, involving metal workers, patients who use power tools, mechanics who work under automobiles and individuals who work with wood or horticulturists. Although the use of safety glasses has increased, a study found that 45% of patients presenting with metallic foreign bodies actually did use eye protection.[20] A thorough ocular examination is always necessary, including lid eversion, to detect any retained material.
Foreign bodies are generally divided into two classes: organic and inorganic. Organic foreign bodies pose an increased risk of infection as they potentially pose a greater threat of contamination with bacteria and fungi. However, it should be noted that inorganic foreign bodies can also carry microbes; therefore, prophylactic topical antibiotics should be used with all types of ocular foreign bodies.
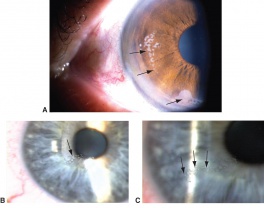
In a study of 288 patients with a corneal foreign body, only 1 patient was associated with a corneal penetration.[21] Even if unlikely, it is imperative to ensure that no foreign body penetrated the cornea by performing an extensive examination of the anterior chamber, iris, lens and posterior pole. Intraocular hemorrhage, vitreous in the anterior chamber, new iridotomy and signs of a violated lens capsule are all an evidence of an intraocular foreign body. High velocity foreign bodies may pass completely through the eye and extraocular diagnostic testing, such as gentle ocular ultrasound, X-ray, CT and possibly MRI (in non-metallic foreign body cases) should be done for localization of the foreign object.
Patients may experience minor or severe discomfort from corneal foreign bodies. Typically, topical ophthalmic anesthetics will provide adequate pain relief for a thorough examination and foreign body removal. Superficial corneal foreign bodies can be removed at the slit lamp with a fine needle, jewelers forcepts, or other ocular instrument. If there is a residual rust ring, it can be effectively removed with a rust ring remover. Certain foreign bodies, such as glass, stone, plastic, and certain metals are not toxic and do not induce inflammation. Therefore, they can be left in place if they are in a stable position, without any risk of further tissue damage, and if the risk of further tissue damage from removal outweighs the risk of leaving the inert foreign body in place. Iron and copper are toxic at the cellular level, can induce an inflammatory tissue reaction and must be removed. Foreign bodies that are deeply embedded or that are perforating the cornea may be removed in the operating room. The removal of any foreign body should be accompanied by irrigation of the wound if retained material is present.
Antibiotics should be initiated prophylactically. If there is an infiltrate associated with the foreign body, cultures should be performed. If the foreign body was organic in nature, cultures should be considered and the patient should be monitored closely for development of an infectious keratitis. It may be difficult to distinguish between an infiltrate and localized corneal edema but the difference can usually be determined with a detailed slit lamp examination, corneal OCT or confocal microscopy.
Because the full extent of a penetrating anterior segment injury can be difficult to appreciate when the anatomy becomes significantly deformed, diagnostic imaging (X-ray, CT and possibly MRI) can be considered.[22] If the patient has a possible metallic foreign body, an MRI should not be performed because of the probability that the strong magnetic fields generated by the MRI device will cause the metallic foreign body to move, which could cause additional tissue damage.
If there is any suspicion of a penetrating or perforating ocular injury, a shield should be applied over the eye. Care must be exercised to avoid any pressure on the globe. The patient should be told to avoid any eye rubbing or squeezing. If the patient is squeezing their eye lids, a facial block, such as an Atkinson - van Lint and/or O’Brien, can be considered at the time of the initial evaluation.[23] Avoid any pressure on the globe, if possible, during the examination and from diagnostic tests, such as a B-scan. It may be possible to obtain additional information about the state of the globe from non-contact testing, such as OCT, CT and MRI. Consider tetanus prophylaxis with metal-related injuries.
Trauma to the Anterior Chamber & Lens
Hyphema
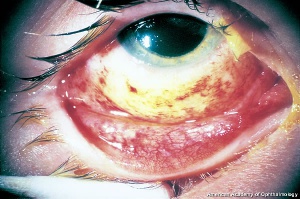
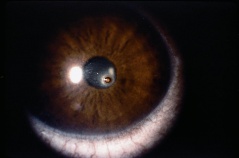
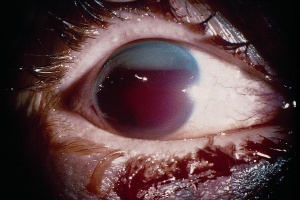
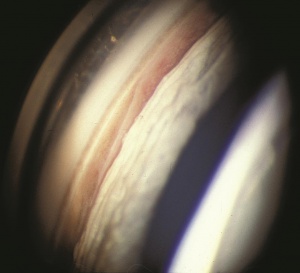
Hyphema, or blood in the anterior chamber, is one of the most common sequelae of blunt trauma to the eye. The hemorrhage is caused by a tear of the iris tissue and/or ciliary body during injury, and occurs at the anterior aspect of the ciliary body in 71% of cases.[24] Patients with hyphema typically complain of decreased visual acuity, pain and photophobia. The diagnosis is made by direct visualization of layered or dispersed blood in the anterior chamber. A microhyphema occurs when there is no layering of blood, but red blood cells are seen within the anterior chamber. An “8-Ball Hyphema” is the result of a large amount of clotted, deoxygenated blood in the anterior chamber, which has a purple/black appearance. A useful way for the examiner to monitor and grade the hyphema is by measuring its vertical height of the layered blood. This measurement can be repeated at each follow-up visit to monitor for resolution. Secondary hemorrhage, or re-bleed, due to lysis of the previously formed blood-clot typically occurs between 2 and 5 days after the initial injury in 25% of patients with hyphema.[25] A rebleed is signified by an increase in hyphema size and is associated with an overall worse visual prognosis.[26]
Because hyphema may accompany severe injury to the eye, a complete examination of the anterior and posterior segment is essential in order to rule out associated injuries and globe rupture. B-scan ultrasonography is often necessary, as the posterior segment may be obscured by blood, and a CT scan of the orbits may be indicated if the suspicion for open globe is high. Gonioscopy should be performed to evaluate the extent of injury to angle structures, but is usually delayed until after the high risk, re-bleed period. Anterior segment OCT may be useful in order to visualize the anterior chamber angle without direct contact.
Elevated intraocular pressure occurs in 32% of patients with hyphema, and patients with preexisting glaucoma are at an increased risk.[24] In the acute period, elevated intraocular pressure is the result of red blood cells and fibrin blocking the trabecular meshwork. Persistent inflammation, resulting in peripheral anterior or posterior synechiae, can lead to delayed angle closure glaucoma and can be prevented by administering topical corticosteroids and cycloplegics. Optic nerve damage, resulting from an acute rise in intraocular pressure, is most likely to occur if the intraocular pressure remains 50mm Hg or greater for 5 days or 35mm Hg or greater for 7 days.[27] Therefore, medications may be required to control the intraocular pressure. Anterior chamber washout or surgical evacuation of the hyphema may be indicated if the pressure elevation persists despite medical management. Special consideration must be given to patients with sickle cell disease, as the sickle-shaped red blood cells cannot effectively pass through the trabecular meshwork, leading to higher intraocular pressures for longer periods of time. Sickle cell disease also poses an increased risk of optic atrophy, which can occur with intraocular pressures less than 35 mm Hg.[28] It is essential to obtain a sickle cell prep and hemoglobin electrophoresis in all patients with hyphema, as sickle cell patients may require an earlier surgical intervention. These patients are typically advised to avoid carbonic anhidrase inhibitors to reduce intraocular pressure.
Corneal blood staining occurs when hemoglobin and its breakdown-products deposit in the cornea, resulting in stromal staining and endothelial dysfunction with subsequent vision loss. It occurs in 2-11% of patients with hyphema and is most common in cases of total hyphema, elevated intraocular pressure, re-bleeding, and in patients with prior endothelial dysfuntion.[29] Early signs of corneal blood staining, such as yellowish discoloration and granules in the posterior stroma are important to recognize, particularly in children who are at risk for developing amblyopia. Anterior chamber wash out may be necessary to avoid this complication. Clearance may occur without intervention, but it starts peripherally and moves centrally with complete resolution taking up to several years. Corneal transplantation may be necessary for a more rapid visual recovery.
Traumatic Iritis
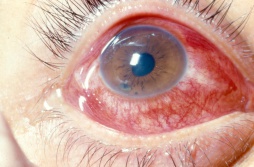
Traumatic Iritis, or anterior chamber inflammation following blunt ocular trauma, is one of the most common causes of anterior uveitis, particularly in the pediatric population. The inflammatory response is caused by prostaglandin release, cell necrosis of the iris and/or ciliary body leading to increased vascular permeability and the influx of white blood cells and protein. Patients present with photophobia, tearing and decreased vision, and physical examination will reveal anterior chamber cell and flare. Irritation of the iris and its attachment to the anterior ciliary body causes spasm of accommodation, sustained miosis and is often associated with a poorly reactive pupil. Less commonly, a mydriatic pupil is found, when the injury causes an iris sphincter muscle tear. Intraocular pressure is often low due to decreased aqueous production from ciliary body shock; however, it can sometimes be elevated due to damage to the trabecular meshwork or due to blockage by inflammatory debris. A rise in intraocular pressure can cause a secondary glaucoma, and if it persists, can lead to optic neuropathy. Traumatic iritis is usually a self-limited entity and usually resolves on its own within 7 to 14 days; however, topical corticosteroids and cycloplegics are routinely initiated to avoid complications associated with prolonged inflammation.
Iris Trauma
Injury to the iris can range from minor, temporary damage to its nerves and muscles to severe structural injury with partial or complete loss of iris tissue. Iris injury frequently results in pupillary abnormalities, and may cause mydriasis, corectopia or polycoria. An abnormal pupil will interfere with the eye’s ability to focus light, leading to visual acuity changes and light sensitivity. If the injury involves the peripheral iris, the anterior chamber angle may be affected and hypotony or glaucoma can result. In order to characterize the extent of iris injury, a slit lamp examination should be performed along with gonioscopy, ultrasound biomicropspy (UBM) and/or anterior segment ocular coherence tomography (OCT).
Tears of the iris sphincter muscle commonly occur after blunt trauma, and can result in traumatic mydriasis. Patients may present with a fixed, dilated pupil and a diminished direct or consensual pupillary reaction to light and accommodative stimuli. The pupil may appear irregular and slit lamp examination may reveal small tears at the pupillary margin. While sphincter tears are the most common cause of anisocoria after trauma, it is crucial to rule out less common but potentially life-threatening etiologies such as third cranial nerve involvement or Horner’s syndrome. It is also important to consider central nervous system pathology, particularly in the setting of bilateral mydriasis.
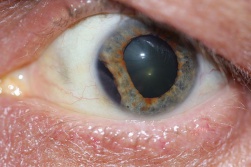
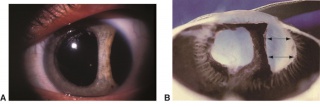
Detachment of the iris root from its insertion site at the ciliary body results in iridodialysis. It frequently occurs after blunt trauma and results in a central D-shaped pupil with a peripheral dark binconvex area where the iris has detached. Associated findings include hyphema, angle recession and peripheral anterior synechiae, and patients can develop chronic open or closed-angle glaucoma. Traumatic aniridia, or complete loss of the iris tissue, can also occur and is usually accompanied by severe intraocular hemorrhage or globe rupture. Aniridia rarely occurs after blunt trauma, but has been reported in eyes that have previously undergone cataract extraction, with the iris exiting a temporarily reopened surgical wound.[30] The iris disinsertion may also be visible in the anterior chamber angle by gonioscopy.
Treatment of iris injury is largely dependent on the severity of glare, diplopia, and decreased visual acuity. Occasionally, unacceptable cosmetic appearance may also be an indication for treatment. Medical management includes the use of miotics and colored contact lenses. Surgical repair of the iris is indicated when a significant visual disturbance is not correctable with medical management alone, when iris diaphragm support is needed for IOL placement, and rarely, for cosmetic reasons. The FDA approved first silicone prosthetic implantable iris device in 2018 (CustomFlex Artificial Iris, HumanOptics) [31]
Angle Recession
Tears between the longitudinal and circular fibers of the ciliary body can occur after blunt trauma and result in recession of the anterior chamber angle. Glaucoma, secondary to angle recession, can occur months to years after the initial injury and presents as a chronic open angle glaucoma with optic nerve damage and visual field loss. Angle recession has been reported to occur in 80% of eyes sustaining blunt trauma, but only 4-8% of eyes with angle recession will develop angle recession glaucoma.[32] Risk factors and related findings associated with the development of glaucoma from angle recession include increased pigmentation of the angle, elevated baseline intraocular pressures, hyphema, lens displacement, and recession involving more than 180 degrees of the angle.
All individuals who have sustained blunt trauma to the eye should undergo gonioscopic evaluation for angle recession, which is classically identified as a widened ciliary body band. Other findings include absent or torn iris processes, a white glistening scleral spur, depression in the overlying trabecular meshwork, and peripheral anterior synechiae. Patients with recessed angles should be routinely monitored with intraocular pressure checks and adjunctive glaucoma testing.
A related anterior chamber angle injury should also be considered in the setting of blunt trauma and a low intraocular pressure. [33] A cyclodialysis cleft can occur when the longitudinal fibers of the ciliary muscle separate from the scleral spur. This results in the drainage of aqueous humor directly into the suprachoroidal space, which may result in hypotony.
Traumatic Cataract and Lens Dislocation
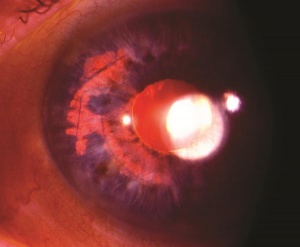
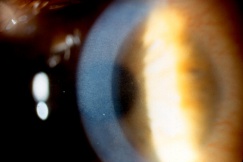
Injury to the crystalline lens is a common occurrence following ocular trauma and can range from small focal opacifications to complete cataract formation. In addition to lens opacities, blunt trauma to the eye can lead to zonular dehiscence, causing ectopia lentis, or displacement of the crystalline lens. The lens may be subluxed (partially displaced) while remaining in the pupillary space, or it may be luxed (completely dislocated) into the anterior chamber or vitreous cavity. Traumatic aphakia, or expulsion of the lens from the eye, can occur in severe penetrating injuries.
Symptoms of lens subluxation or dislocation depend on the degree of zonular injury and lens displacement. If the subluxation is minimal, visual acuity may not be affected or it may be mildly decreased secondary to lenticular astigmatism. Patients may complain of glare or monocular diplopia. Greater degrees of subluxation and dislocation out of the visual axis can cause a severe decrease in visual acuity. Iridodonesis (quivering of the iris) and/or phakodonesis (quivering of the lens) may be noted on slit lamp examination and signal zonular loss. The edge of a subluxed lens may be visible through the dilated pupil. The dislocated lens may be found in the anterior chamber, floating in the vitreous, or laying on the retina. The location of the dislocated lens can also be visualized by slit lamp or with B-scan, CT and high resolution MRI scan of the globes. If a mild traumatic injury leads to lens subluxation, then systemic conditions that cause weak zonules should be considered, such as syphilis, Marfan’s sundrome, Weill-Marchesani syndrome, homocystinuria, and pseudoexfoliation syndrome.[34]
Traumatic lens injury may present as an acute, subacute, or late sequela of ocular trauma and is one of the major causes of acute or long-standing visual loss after injury to the eye. Cataracts and zonular dehiscence following blunt trauma are the result of coup (direct) and contrecoup (indirect) injury to the lens.[35] The resulting traumatic shockwave and equatorial expansion of the globe causes injury to both the anterior and posterior lens structures leading to capsular and zolunlar disruption and cataract which may be stable or progressive. Coup injury is also responsible for the imprint of pigment on the anterior lens capsule known as the Vossius Ring that is frequently seen in these cases. Penetrating trauma or intraocular foreign bodies can lacerate the anterior lens capsule, leading to focal cortical changes, or rapid lens opacification. The lens material that maybe released after both blunt and penetrating injury can lead to severe intraocular inflammation and elevation of intraocular pressure. [36]
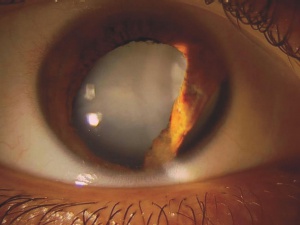
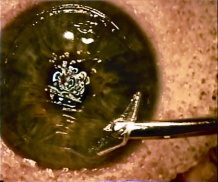
Patients with traumatic cataract complain of decreased vision, glare and monocular diplopia. While a traumatic cataract may result immediately following the injury, it may also develop gradually over weeks, months or years. Some traumatic cataracts remain localized and stable, while others may progress to total lens opacification. The traumatic cataract is classically described as subcapsular and star-shaped, and is known as a Rosette Cataract. Other types of cataract, such as a mature white cataract can also be seen. Lens swelling and the integrity of the anterior and posterior capsule should be documented and, in the acute setting, the severity of lens-associated intraocular inflammation should be graded.
The most common vision threatening complication of lens injury is glaucoma, which can occur from a variety mechanisms. The release of macroscopic lens particles through a capsular rupture and into the anterior chamber can obstruct aqueous outflow and cause lens-induced glaucoma. Clinical findings include elevated IOP and visible lens material in the anterior chamber or angle. Anterior chamber inflammation is common, but may not be present. Phacoantigenic glaucoma occurs when released lens material causes a severe granulomatous immune reaction. Clinical findings include anterior chamber inflammation with keratic precipitates (KPs) in addition to elevated intraocular pressure. Pupillary block can occur when the lens completely occludes the pupil, which prohibits the flow of aqueous humor from the ciliary body to the anterior chamber angle. This condition results in a flat anterior chamber with a high posterior chamber intraocular pressure. Also, a subsequent angle closure glaucoma can occur if the lens dislocates into the anterior chamber. Other secondary causes of elevated intraocular pressure in the setting of lens injury includes peripheral anterior and posterior synechiae and other anterior chamber angle injuries.
Traumatic cataracts may be observed even if the visual acuity is not compromised. Focal opacities of the peripheral lens causing glare and monocular diplopia may be managed with topical miotic agents. Emergent or urgent surgical removal of the lens may be needed in the case of pupillary block glaucoma caused by the lens dislocating into the anterior chamber, or in cases of lens particle induced or phacoantigenic glaucoma. [37] Surgical timing is also important to consider in a child, in whom amblyopia is a concern. Despite the timing of cataract extraction, the surgeon should be prepared to manage zonular dehiscence and anterior vitreous prolapse.
Post-Surgical Anterior Segment Trauma After Cataract and Refractive Surgery
Refractive surgery utilizes different modalities to modify the refractive power of the cornea and lens, thereby correcting the refractive state (i.e. myopia, hyperopia, astigmatism, and presbyopia) of the eye. Intraocular refractive surgery such as cataract (or refractive lens exchange) and phakic intraocular lens (pIOL) surgery corrects ametropia with implantation of an intraocular lens (IOL). Refractive corneal procedures can be either incisional, lamellar, photoablative laser, or intra-stromal, and they all inherently weaken the native cornea. This inherent weakness, from corneal surgery or surgical incisions for intraocular surgery, may be exposed when the eye suffers a traumatic injury.
In ocular trauma patients, the diagnosis and treatment of life-threatening injuries takes precedence over ophthalmic trauma. While a careful history regarding the nature of the injury is important, even mild or benign appearing trauma may be more damaging in a post-surgical eye.
Intraocular Refractive Surgery
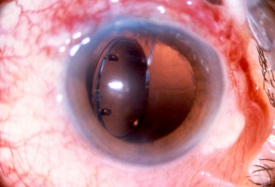
Refractive lens exchange and cataract surgery remove the crystalline lens and replace it with an IOL. Prior to the phacoemulsification era, extracapsular cataract (ECCE) surgery involved a large limbal incision or scleral tunnel. While ECCE incisions were historically as large as 10 mm in chord length, phacoemulsification cataract surgery employs a clear corneal incision (or narrow scleral tunnels), which typically ranges between 2.2 to 2.75 mm. Phakic IOLs (pIOL) are able to correct ametropia by lens insertion into 3 anatomical locations: anterior chamber iris fixated (Artisan/Verisyse), anterior chamber angle supported (Kelman Duet, Acrysof Cachet), or posterior chamber with no fixation (Implantable Collamer Lenses).[38][39] Incisions from these surgeries produce a potential area of weakness that may lead to wound dehiscence after trauma. Larger incisions from traditional ECCE are more likely to suffer dehiscence than smaller phacoemulsification or pIOL incisions.[40][41] Prior cataract surgery may stress the zonular support, and subsequent trauma may dislocate the implanted IOL. Ruptured ECCE wounds account for a significant proportion of all open globe injuries, and they are associated with a high possibility of retinal damage (e.g. choroidal hemorrhages and retinal detachment), often leading to a poor visual outcome. Dehiscence of the phacoemulsification wound is less common and is more frequently associated with a better visual prognosis. Other pathology related to trauma may involve IOL dislocation within the eye from an associated zonulopathy or capsular tear. The IOL may also be partially or completely expelled from globe through a weakened area (i.e. prior incisions).
Although uncommon, traumatic dislocation of pIOLs has been reported. Complete aniridia, with expulsion of the crystalline lens and vitreous through the 6 mm surgical wound has been reported 6 months following Artisan lens implantation (anterior chamber iris fixated).[42] It was hypothesized that the trauma caused the enclavated lens to shear the iris from its root thereby creating a complete iridodialysis, resulting in traumatic aniridia. Partial aniridia has also been reported.[43] Decentration/dislocation of the pIOL is also a potential sequela of trauma. Additionally, cataract formation may occur with pIOLs and is conceivably more common following trauma, especially with posterior chamber positioned pIOL. There is also a risk of endothelial cell damage and resulting corneal decompensation with traumatic IOL dislocations.
There are still a significant number of the pseudophakic elderly individuals, who underwent traditional ECCE. While all incisions should be carefully inspected, a history of ECCE should prompt attentive inspection of the ECCE wound. There should be a low threshold to perform exploration of the globe, in order to exclude a potentially dehisced cataract wound, particularly in the setting of bullous subconjunctival hemorrhage.[40] Fluorescein can be used to check for Seidel test positivity of suspicious wounds. Examination of the IOL or pIOL, after trauma, may require head positioning to identify displacement, dislocation or significant pseudophakodonesis (signs of zonular trauma damage). Slit lamp examination in patients with a history of pIOL implantation must be performed with special attention to the haptic enclavation sites on the mid-peripheral iris for iris-fixated pIOLs and to the haptic position for the other pIOL types. In all cases of trauma, after cataract surgery, the cornea should be examined for edema, which may be suggestive of endothelial cell damage. Intraocular pressure must be measured, and a gonioscopic examination should be performed, if possible, given the patient’s risk of peripheral anterior synechae and potential iridodialysis or cyclodialysis.[44] Pupil irregularity, iris atrophy, iris tears and iris transillumination defects should be noted and may be associated with pupillary ovalization.
An irregular pupil can also be indicative of a dislocated IOL and /or a partially extruded IOL. Iris atrophy (sectoral) or transillumination defects may signify trauma from a dislocated IOL that is now in contact with the iris. The slit beam must also be directed toward the pIOL and natural lens to assess the distance between the posterior surface of the pIOL and the anterior surface of the crystalline lens. pIOL/lens touch following trauma may also be associated with increased risk of cataract formation. The anterior chamber should also be evaluated for hyphema as well as cell and flare.
Incisional Refractive Surgery
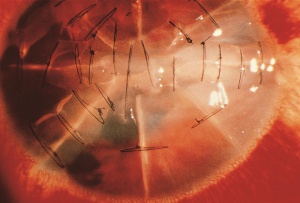
Radial keratotomy (RK), performed for myopia, utilized a series of radial incisions into the paracentral and peripheral anterior cornea to decrease the refractive power of the cornea.[45] The incisions were intended to reach a specific depth within the cornea (ideal depth between 85% - 90%) and ranged between 4 and 32 in number. Studies of the structure and strength of these wounds demonstrated an early bed of corneal epithelial cells (epithelial plug) that grew into the incision. It is thought that the persistence of the epithelial plugs make the incisions more susceptible to rupture from blunt trauma.[46][47] Both longer and deeper (>70% depth) RK incisions have been shown to require less traumatic force to rupture.[48][49][50] Even among the estimated 2-3 million eyes that have undergone RK, wound rupture appears to be relatively uncommon.[51] Based on animal models, the most common rupture pattern was a "cut-to-cut" rupture, in which two RK incisions were connected by the rupture site.[52] This was followed in frequency by a radial corneoscleral laceration along an RK incision, a stellate rupture (3 or more RK incisions rupture and intersect to form a single laceration), and an "incision-opening" rupture involving a single RK incision without extension.[53] After trauma, ruptured RK wounds tend to extend across the visual axis in over 70% of the eyes, given the close proximity of incisions to the visual axis and the thinner corneal tissue in this area.[54] Despite the risk of dehiscence/rupture, the remaining 5-10% of non-incised corneal stroma with Descemet's membrane appears to afford a relatively strong barrier to wound rupture.[55] The degree and distribution of force may play a role in whether incisions rupture or just dehisce; [55] additionally, there appears to be a decreased risk of rupture after 3 to 4 years because of wound remodeling.[56]
Evaluation requires a complete general and ophthalmic examination. Vigilant examination with a narrow slit beam can help determine the depth of the incision and/or injury. If not obvious, a Seidel test with fluorescein can confirm one’s suspicion of a perforating corneal injury. A variety of microorganisms have been found to cause incisional keratitis. Incisions should be carefully monitored for infiltrates, accompanied with initial cultures and aggressive treatment with broad spectrum anti-microbials, if involved after ocular trauma. Epithelial downgrowth, also called epithelial ingrowth, after RK is rare but can occur if deep radial incisions splay open during trauma.[57] [58] This may result from micro-perforation with implantation of epithelial cells into the anterior chamber from a preexisting epithelial plug that rapidly self-sealed. [53] If suspected, confocal microscopy permits noninvasive, in vivo microscopic imaging to diagnose epithelial downgrowth. Argon laser photocoagulation can be used to confirm the diagnosis of epithelial downgrowth in cases with iris involvement.
Despite no obvious trauma to the incisions, the RK incisions may still be affected. Forstot reported a case of moderate, direct trauma that caused a subconjunctival hemorrhage and traumatic iritis.[58] Although there was no visible dehiscence of the RK incisions, they measured an increased keratometric cylinder of 1.5 diopters, which regressed spontaneously. Topography and a careful manifest refraction can help detect these changes.
Trauma has been found to be a risk factor for late-onset incisional infectious keratitis. Incisional keratitis may present as an infiltrate along the length of the incision, conjunctival injection, surrounding corneal edema, with or without an anterior chamber reaction or hypopyon. Pseudocysts within keratotomy wounds may be a predisposing factor, as they may permit corneal stromal access of bacteria or occasionally break down, resulting in microscopic or macroscopic erosions.[59] Finally, incisional infections can potentially lead to endophthalmitis.
Astigmatic Keratotomy
Corneal astigmatism can be addressed by astigmatic keratotomy with incisional and excimer laser ablative methods. Options such as tangential (horizontal/linear) keratotomy, arcuate keratotomy (AK), and limbal relaxing incisions (LRI) are corneal relaxing incisions made parallel to the limbus that lead to flattening in the meridian of the incision and steeping in the meridian 90 degrees away. With tangential and arcuate incisions, the incisions are placed closer to the visual axis compared to a LRI incision. Based on nomograms, tangential and AK incisions are made approximately 95% depth within the cornea. For LRIs, the usual depth is 600 microns (range 450 - 650 microns) or 50 microns less than the thinnest limbal corneal thickness. Wound healing after astigmatic keratotomy appears similar to the healing process following standard radial keratotomy, with the exception that AK incisions exhibit faster wound healing.[60] This was attributed to the better apposition of tangential incisions while semi-radial/radial incisions tend to gape as a result of the mechanical stress of intraocular pressure. Tangential incisions were also found to have a shallower depth, compared to RK, which results in greater structural integrity of the cornea.
There are reports of astigmatic keratotomy wound rupture although this has only been reported in association with RK wound rupture.[61][62] There appears to be a much lower risk of isolated rupture of astigmatic keratotomy incisions or LRIs; however, incisions from AK are at risk for late-onset keratitis following trauma, similar to RK incisions.
Intrastromal Corneal Ring Segments (ICRS)
Intrastromal corneal implants have evolved from complete ring insertion to the insertion of two C-shaped rings. Although the initial indication for the ICRS procedure was low myopia, ICRSs have been adopted for the treatment of corneal ectasia following LASIK as well as severe keratoconus for treatment of irregular astigmatism and corneal instability.[63][64][65]
If performed manually, a one millimeter radial incision at 60-80% of the corneal thickness is created with a diamond blade knife along the steepest meridian. Two continuous stromal tunnels are created for the insertion of the intrastromal ring segments. It is possible for the intrastromal ring segments to migrate with trauma, superimposing the distal segments. Trauma can also cause extrusion of the segments and theoretically could cause the segments to penetrate the anterior chamber or perforate the anterior corneal stroma. In addition, patients with ICRS should be examined for malpositioned ring segments; including overriding segments, extruded segments, and segments penetrating the anterior chamber.
Laser-Based Refractive Surgery
Photorefractive keratectomy (PRK) utilizes the excimer laser to change the shape of the cornea. Studies have demonstrated that PRK does not significantly weaken the structural integrity of the cornea.[66][67] PRK in porcine eyes did not affect ocular integrity after blunt trauma when subjected to a squash ball axially impacting the cornea.[68] While most clinical PRK protocols do not exceed 15% of corneal thickness, in vitro studies have demonstrated rupture at ablation sites with greater than 35-40% corneal thickness.[66][67] There are no reported cases of isolated globe rupture after PRK treatment to date.
Laser in-situ keratomileusis (LASIK), first approved in 1999, is one of the most commonly performed refractive procedures to correct various degrees of myopia, hyperopia, and astigmatism. The biomechanical properties of the post-LASIK cornea are primarily determined by the residual stromal bed with the LASIK flap only contributing minimally to the tectonic corneal strength and stability.[69][70] [71][72] Although requiring less energy to rupture, compared with that of normal eyes, studies have demonstrated the level of energy required to rupture both PRK and LASIK eyes was much greater than RK eyes.[73]
While LASIK does not appear to structurally weaken the globe, post-LASIK ectasia may predispose the patient to corneal rupture from trauma.[74] Traumatic dislocations of the LASIK flap can occur even years following surgery; however, LASIK flaps require a substantial amount of force to create a dehiscence. Femtosecond laser created flaps heal with more fibrosis at the flap margin, compared to their microkeratome created flap counterparts, which imparts increased structural integrity.[75] Patients may present with either a partially dislocated LASIK flap or a completely detached flap. Traumatic flap dislocation may occur in up to 1% of patients and can predispose patients to a host of other subsequent complications, such as epithelial ingrowth, diffuse lamellar keratitis, infectious keratitis, and interface foreign bodies.[76]
LASIK Flap Avulsion and Tears
Compared to flap dislocations, a flap avulsion is much less common.[77] The degree of trauma required to completely detach a flap, generally involves a shearing force at a particular angle to sever the hinge. Irregular astigmatism may result after the injury, rigid gas permeable lens fitting may be necessary to optimize visual acuity after re-epithelialization. It should be noted there is increased risk of corneal haze (fibrosis), which can occur after this type of injury and mitomycin C may be used off-label to reduce the risk of corneal haze formation.
If not completely avulsed, a flap tear can result in the displacement of the flap, which is associated with epithelial ingrowth at the areas of exposed stroma.[78] The exposed stromal bed may also develop opacities that are visually significant. Slit lamp and anterior segment optical coherence tomography (OCT) can be valuable in assessing flap dislocations. OCT’s high magnification and resolution can identify the orientation of the flap and can reveal the location of epithelial ingrowth.
LASIK Corneal Penetrating/Perforating Injuries
When corneal injuries occur in patients with a history of LASIK, one must evaluate how both the flap and the stromal bed have been affected. Careful slit lamp examination can help determine partial and full-thickness injuries. The Seidel test may be falsely negative in a corneal perforation if the flap is slightly offset from the stromal bed because the aqueous fluid may accumulate in the flap interface and may not be apparent on the epithelial surface.
Interface Fluid Syndrome (or Pressure-Induced Stromal Keratopathy [PISK])
Lamellar interface fluid accumulation is a well-described LASIK complication described by Lyle and Jin that is typically seen following corticosteroid-related intraocular pressure (IOP) elevation.[79] Here, fluid collects in the interface potential space as aqueous humor diffuses into the corneal stroma in the setting of compromised endothelium. Interface fluid syndrome has also been reported following blunt, penetrating/perforating, and barotrauma.[80][81][82] The time from ocular trauma to development of interface fluid syndrome is much shorter (~2-3 days) than that associated with corticosteroids (typically after 10-21 days of use). Anterior segment OCT may prove valuable as the interface fluid may masquerade as interface hyperreflection (haze)/keratitis on slit lamp examination. In blunt trauma, as there is no direct pathway for fluid to sequester in the interface, it has been hypothesized that direct traumatic force to the flap and anterior chamber inflammation contribute to the formation of interface fluid.[80][81][82]
Diffuse Lamellar Keratitis
Diffuse lamellar keratitis (DLK) is a well-known complication of LASIK, first described by Robert Maddox as “Sands of Sahara Syndrome,” and it was later termed “DLK” by Smith and Maloney.[83] DLK classically develops 2 to 6 days following LASIK and typically resolves 5-8 days after the initiation of appropriate therapy (corticosteroids with or without lifting the flap).[84] [83] DLK can occur months to years after LASIK surgery with trauma being one the more common triggering etiologies. Even minor corneal trauma can result in DLK. This is especially true if the LAIK flap is involved. This demonstrates the persistence of the potential space made upon flap creation, allowing endogenous inflammatory cells to collect in this space.[85]
Epithelial Ingrowth
Epithelial ingrowth is a complication in which corneal epithelial cells advance under the LASIK flap or are trapped within the lamellar interface. It can be an early postoperative complication or can accompany trauma involving full or partial dehiscence of the corneal flap.[86] Epithelial ingrowth typically advances from the periphery where the flap was disrupted; however, any corneal penetrating/perforating injury along the flap can be associated with epithelial ingrowth in that area.
Flap Folds
Flap folds (or striae) can appear within the first 24 hours after LASIK, but most occur within the first week. There have been cases of striae reported without corneal flap dislocation following trauma years after the initial surgery.[87] It is important to consider the formation of striae when examining LASIK patients with ocular trauma even in the absence of flap slippage, subluxation, or dislocation. Fluorescein dye can be helpful to detect flap folds. An inflammatory reaction and asymmetrical hyperplasia (with epithelial growth) can fix the folds into place, which leads to persistent visual deficits, which are difficult to correct.[88] Therefore, LASIK flap stria should be addressed and removed as soon as possible.
Infectious Keratitis (IK)
Trauma increases the risk of IK in patients who have previously undergone LASIK. An epithelial break occurring any time after LASIK may allow microorganisms to reach the flap interface.[89] An infection may progress more rapidly as it can spread within the interface potential space. Interface infections can be more difficult to treat as the microorganisms are protected from the natural ocular surface defenses, and the antimicrobials do not as penetrate well.[90] Any infiltrate after LASIK should be considered infectious until proven otherwise as IK can have very severe complications, including decreased visual acuity, pain, flap melting, astigmatism, and corneal scarring. Infectious keratitis may be differentiated from DLK in that the latter is usually confined to the plane of the flap creation, whereas infectious keratitis can extend above and below the interface and appear as an area of focal inflammation. Trauma can be a risk factor for late onset infections, especially fungal keratitis,[90],[91] but atypical mycobacterium and acanthamoeba must also be considered in this setting.[92] A thorough history and nature of the ocular trauma can help the examiner determine the risk factors for bacterial, fungal, amoebic and other infectious agents.
Interface Foreign Bodies
As described earlier, the flap-stromal interface is vulnerable given the limited healing that occurs. While flap dislocation or penetrating cornea trauma introduces an entrance into this potential space for foreign body debris, another possible mechanism involves sharp ocular trauma that may leave foreign body debris in the LASIK interface. Interface debris can also incite an inflammatory reaction.
Small Incision Lenticule Extraction (SMILE)
Small Incision Lenticule Extraction (SMILE) is a recently developed femtosecond laser based refractive surgery procedure that evolved from the Femtosecond Lenticule Extraction technique introduced in 2007. In the human cornea, the anterior stroma is known to have greater biomechanical strength than the posterior lamellae. The intrastromal incisions of SMILE are less invasive than alternative refractive surgical procedures, leaving the anterior stroma and Bowman layer intact. Therefore, a theoretical advantage to SMILE is increased corneal stability and stromal tensile strength.[93] Studies by Agca et al, Wu et al, and others have compared corneal hysteresis and corneal resistance factor as parameters of relative corneal biomechanical strength following SMILE and LASIK; however, the results have been inconclusive.[93][94][95][96]
SMILE essentially eliminates flap-related concerns such as dislocation and avulsion, since there is no flap; however, there is still a potential space that is created. Post-trauma complications such as DLK, IK, epithelial ingrowth, and foreign bodies are still concerns.[96] There are limited case reports concerning post-traumatic complications as SMILE is a relatively new technique.
Video: https://www.aao.org/annual-meeting-video/refractive-lenticule-extraction-complications
Conclusion
Trauma can affect the eye’s native structure in unique ways. The astute clinician must be aware of these potential trauma related ocular injuries and may need to utilize different techniques to examine the eye. Early and thorough identification of the affected anterior segment tissues involved will help with prompt and appropriate management.
Additional Resources
- Corneal Abrasion. American Academy of Ophthalmology. EyeSmart/Eye health. https://www.aao.org/eye-health/diseases/corneal-abrasion-7. Accessed March 28, 2019.
- Porter D, Jimenez EM. Corneal Laceration. American Academy of Ophthalmology. EyeSmart/Eye health. https://www.aao.org/eye-health/diseases/corneal-laceration. Accessed March 28, 2019.
References
- ↑ Vajpayee RB, Gupta NK, Angra SK, et al. Contact thermal burns of the cornea. Can J Ophthalmol. 1991;26(4):215–8.
- ↑ Boone KD, Boone DE, Lewis RW 2nd, Kealey GP. A retrospective study of the incidence and prevalence of thermal corneal injury in patients with burns. J Burn Care Rehabil. 1998;19(3):216–8.
- ↑ Mannis MJ, Miller RB, Krachmer JH. Contact thermal burns of the cornea from electric curling irons. Am J Ophthalmol. 1984;98(3):336–9.
- ↑ Shimazaki J, Konomi K, Shimmura S, Tsubota K. Ocular surface reconstruction for thermal burns caused by fireworks. Cornea. 2006;25(2):139–45.
- ↑ Sharifipour F, Baradaran-Rafii A, Idani E, et al. Oxygen therapy for acute ocular chemical or thermal burns: a pilot study. Am J Ophthalmol. 2011;151(5):823–8.
- ↑ Kalwerisky K, Davies B, Mihora L, et al. Use of the boston ocular surface prosthesis in the management of severe periorbital thermal injuries: a case series of 10 patients. Ophthalmology. 2012;119(3):516–21.
- ↑ Jump up to: 7.0 7.1 7.2 Schein OD. Phototoxicity and the cornea. J Natl Med Assoc. 1992;84(7):579–83.
- ↑ Jump up to: 8.0 8.1 8.2 8.3 8.4 8.5 Rafailov L, Lazzaro DR. Ch 3. Corneal Trauma. Kaufman SC, Lazzaro DR (Editors) Textbook of Ocular Trauma (1st ed). Springer, Cham, Switzerland, 2017
- ↑ Morgan SJ. Chemical burns of the eye: causes and management. Br J Ophthalmol. 1987;71(11):854–7.
- ↑ McCulley JP. Ocular hydrofluoric acid burns: animal model, mechanism of injury and therapy. Trans Am Ophthalmol Soc. 1990;88:649–84.
- ↑ Dua HS, King AJ, Joseph A. A new classification of ocular surface burns. Br J Ophthalmol. 2001;85 (11):1379–83.
- ↑ Kuckelkorn R, Kottek A, Reim M. Intraocular complications after severe chemical burns—incidence and surgical treatment. Klin Monbl Augenheilkd. 1994;205(2):86–92.
- ↑ Wagoner MD. Chemical injuries of the eye: current concepts in pathophysiology and therapy. Surv Ophthalmol. 1997;41(4):275–313.
- ↑ Jump up to: 14.0 14.1 Schrage NF, Langefeld S, Zschocke J, et al. Eye burns: an emergency and continuing problem. Burns. 2000;26(8):689–99.
- ↑ Rihawi S, Frentz M, Schrage NF. Emergency treatment of eye burns: which rinsing solution should we choose? Graefe’s Arch Clin Exp Ophthalmol. 2006;244(7):845–54.
- ↑ Edwards RS. Ophthalmic emergencies in a district general hospital casualty department. Br J Ophthalmol. 1987;71(12):938–42.
- ↑ Lin YB, Gardiner MF. Fingernail-induced corneal abrasions: case series from an ophthalmology emergency department. Cornea. 2014;33(7):691–5.
- ↑ Ball DC, Bouchard CS. Ocular morbidity associated with airbag deployment: a report of seven cases and a review of the literature. Cornea. 2001;20(2):159–63.
- ↑ Roth S, Thisted RA, Erickson JP, et al. Eye injuries after nonocular surgery. A study of 60,965 anesthetics from 1988 to 1992. Anesthesiology. 1996;85(5):1020–7.
- ↑ Ramakrishnan T, Constantinou M, Jhanji V, Vajpayee RB. Corneal metallic foreign body injuries due to suboptimal ocular protection. Arch Environ Occup Health. 2012;67(1):48–50.
- ↑ Luo Z, Gardiner M. The incidence of intraocular foreign bodies and other intraocular findings in patients with corneal metal foreign bodies. Ophthalmology. 2010;117(11):2218–21.
- ↑ Hamill MB. Corneal and scleral trauma. Ophthalmol Clin North Am. 2002;15(2):185–94.
- ↑ Schimek F, Fahle M. Techniques of facial nerve block. Br J of Ophthal. 1995; 79: 166-173.
- ↑ Jump up to: 24.0 24.1 Read, J., & Goldberg, M. F. (1974). Comparison of medical treatment for traumatic hyphema. In Transactions of the American Academy of Ophthalmology and Otolaryngology. (5 ed., Vol. 78).
- ↑ Walton, W., Von Hagen, S., Grigorian, R., & Zarbin, M. (2002). Management of traumatic hyphema. Surv Ophthalmol, 47(4), 297-334
- ↑ Rahmani, B., Jahadi, H. R., & Rajaeefard, A. (1999). An analysis of risk for secondary hemorrhage in traumatic hyphema. Ophthalmology, 106(2), 380-385.
- ↑ Hoskins HD. (1978). Secondary glaucoma. In Heilman K, Richardson KT, eds. Glaucoma: Conceptions of a Disease, Pathogenesis, Diagnosis Therapy (pp. 376). Philadelphia: WB Saunders.
- ↑ Nasrullah, A., & Kerr, N. C. (1997). Sickle cell trait as a risk factor for secondary hemorrhage in children with traumatic hyphema. Am J Ophthalmol, 123(6), 783-790.
- ↑ Brodrick JD. Corneal blood staining after hyphaema. Br J Ophthalmol. 1972;56:589–593
- ↑ U.S. Food and Drug Administration. FDA approves first artificial iris. FDA Press News Release. 2018.
- ↑ Salmon JF, Mermound A, Ivey A. The detection of post traumatic angle recession by gonioscopy in a population based glaucoma survey. Ophthalmology. 1994 Nov;101(11):1844-50.
- ↑ Botwinick A., Garg R. Trauma-Induced Glaucoma. Glaucoma Today. May-June 2017.
- ↑ Bruce M. Zagelbaum, P. S. (2008). Anterior Segment Truama. In J. W. Daniel M. Albert MD MS, Albert & Jakobiec's Principles & Practice of Ophthalmology (pp. 5093-5111). Philadelphia: Elsevier.
- ↑ Wolter, J. R. (1963). Coup-contrecoup mechanism of ocular injuries. Am J Ophthalmol, 56, 785-796.
- ↑ Shah MA, Shah SM, Shah SB, et al. Morphology of traumatic cataract: does it play a role in final visual outcome? BMJ Open. 2011;1.
- ↑ Papconstantinou D, et al. Lens-induced glaucoma in elderly. Clin Interv Aging. 2009, 4: 331-336.
- ↑ Knorq MC, Wiesinger B, Liewnann A, et al. Laser in situ keratomileusis for moderate and high myopia and myopic astigmatism. Ophthalmology 1998;105:932-40.
- ↑ Ghanem RC, Allemann N, Azar DT. Phakic Intraocular Lenses. In: Yanoff M, ed. Ophthalmology. 4th ed. Philadelphia, PA: Elsevier Saunders Inc.; 2014:127-40.
- ↑ Jump up to: 40.0 40.1 Kloek CE, Andreoli MT, Andreoli CM. Characteristics of traumatic cataract wound dehiscence. Am J Ophthalmol. 2011 Aug;152(2):229-33.
- ↑ Ball JL, McLeod BK. Traumatic wound dehiscence following cataract surgery: a thing of the past? Eye (Lond) 2001;15(Pt 1):42–44.
- ↑ Lee SJ. Traumatic aniridia and aphakia after Artisan intraocular lens implantation. J Cataract Refract Surg 2007;33:1341-2.
- ↑ Hyun J, Chung JK, Lee SJ. Traumatic partial aniridia and cataract after iris-fixated foldable phakic intraocular lens implantation. Case Rep Ophthalmol 2013;4:210-5.
- ↑ Alió JL, Toffaha BT, Peña-Garcia P, et al. Phakic intraocular lens explantation: causes in 240 cases. J Refract Surg 2015;31:30-5.
- ↑ Fyodorov SN, Durnev VV. Operation of dosage dissection of corneal circular ligament in cases of myopia of a mild degree. Ann Ophthalmol 1979;11:1885-90.
- ↑ Glasgow BJ, Brown HH, Aizuss DH, et al. Traumatic dehiscence of incisions seven years after radial keratotomy. Am J Ophthalmol 1988;106:703-7.
- ↑ Bryant MR, Szerenyi K, Schmotzer H, McDonnell PJ. Corneal tensile strength in fully healed radial keratotomy wounds. Invest Ophthalmol Vis Sci 1994;35:3022-31.
- ↑ Luttrull JK, Jester JV, Smith RE. The effect of radial keratotomy on ocular integrity in an animal model. Arch Ophthalmol 1982;100:319-20.
- ↑ Pinheiro MN Jr, Bryant MR, Tayyanipour R, et al. Corneal integrity after refractive surgery. Effects of radial keratotomy and mini-radial keratotomy. Ophthalmology 1995;102:297-301.
- ↑ Steinemann TL, Baltz TC, Lam BL, et al. Mini radial keratotomy reduces ocular integrity. Axial compression in a postmortem porcine eye model. Ophthalmology 1998;105:1739-44.
- ↑ McDonnell PJ. Sight-threatening complications after radial keratotomy. Arch Ophthalmol 1996;114:211-2.
- ↑ Larson BC, Kremer FB, Eller AW, Bernardino VB Jr. Quantitated trauma following radial keratotomy in rabbits. Ophthalmology 1983;90:660-7.
- ↑ Jump up to: 53.0 53.1 Rylander HG, Welch AJ, Fremming B. The effect of radial keratotomy in the rupture strength of pig eyes. Ophthalmic Surg 1983;14:744-9.
- ↑ Binder PS, Waring GO, Arrowsmith PN, Wang C. Histopathology of traumatic corneal rupture after radial keratotomy. Arch Ophthalmol 1988;106:1584-90.
- ↑ Jump up to: 55.0 55.1 Bouchard CS, Vaziri B. Dehiscence of radial keratotomy wounds without globe rupture following explosion injury. J Refract Surg 2001;17:561-3.
- ↑ Vinger PF, Mieler WF, Oestreicher JH, Easterbrook M. Ruptured globes following radial and hexagonal keratotomy surgery. Arch Ophthalmol 1996;114:129-34.
- ↑ Nemi A, Bahadur RP, Randleman JB. Traumatic epithelial downgrowth after radial keratotomy. J Cataract Refract Surg 2008;34:327-9.
- ↑ Jump up to: 58.0 58.1 Forstot SL, Damiano RE. Trauma after radial keratotomy. Ophthalmology 1988;95:833-5.
- ↑ Heidemann DG, Dunn SP, Chow CY. Early- versus late-onset infectious keratitis after radial and astigmatic keratotomy: clinical spectrum in a referral practice. J Cataract Refract Surg 1999;25:1615-9.
- ↑ Deg JK, Binder PS. Wound healing after astigmatic keratotomy in human eyes. Ophthalmology 1987;94:1290-8.
- ↑ Lee BL, Manche EE, Glasgow BJ. Rupture of radial and arcuate keratotomy scars by blunt trauma 91 months after incisional keratotomy. Am J Ophthalmol 1995;120:108-10.
- ↑ Eggleston RJ. Surgical repair of multiple ruptures of radial and transverse incisions under topical anesthesia. J Cataract Refract Surg 1996;22:1394.
- ↑ Park J, Gritz DC. Evolution in the use of intrastromal corneal ring segments for corneal ectasia. Curr Opin Ophthalmol 2013;24(4):296-301.
- ↑ Rabinowitz YS. Intacs for keratoconus. Curr Opin Ophthalmol 2007;18:279–83.
- ↑ Carrasquillo KG, Rand J, Talamo JH. Intacs for keratoconus and post-LASIK ectasia: mechanical versus femtosecond laser-assisted channel creation. Cornea 2007;26(8):956-62.
- ↑ Jump up to: 66.0 66.1 Campos M, Lee M, McDonnell PJ. Ocular integrity after refractive surgery: effects of photorefractive keratectomy, phototherapeutic keratectomy, and radial keratotomy. Ophthalmic Surg 1992;23:598-602.
- ↑ Jump up to: 67.0 67.1 Burnstein Y, Klapper D, Hersh PS. Experimental globe rupture after excimer laser photorefractive keratectomy. Arch Ophthalmol 1995;113:1056-9.
- ↑ Galler El, Umlas JW, Vinger PF, Wu HK. Ocular integrity after quantitated trauma following photorefractive keratectomy and automated lamellar keratectomy. Abstract presented at: Association for Research in Vision and Ophthalmology, May 16, 1995; Ft. Lauderdale, FL. Abstracts in: Invest Ophthalmol Vis Sci 36[Suppl]:580, 1995.
- ↑ Dawson DG, Kramer TR, Grossniklaus HE, et al. Histologic, ultrastructural, and immunofluorescent evaluation of human laser assisted in situ keratomileusis corneal wounds. Arch Ophthalmol 2005;123:741-56.
- ↑ Schmack I, Dawson DG, McCarey BE, et al. Cohesive tensile strength of human LASIK wounds with histologic, ultrastructural, and clinical correlations. J Refract Surg 2005;21:433-45.
- ↑ Roberts C. The cornea is not a piece of plastic. J Refract Surg 2000;16:407–13.
- ↑ Chan C, Boxer Wachler B. Corneal ectasia and refractive surgery. International Ophthalmology Clinics 2006;46(3):13–25.
- ↑ Peacock LW, Slade SG, Martiz J, et al. Ocular integrity after refractive procedures. Ophthalmology. 1997;104:1079-83.
- ↑ Cheung AY, Heidemann DG. Globe Rupture of a Post-LASIK Keratectasia Eye From Blunt Trauma. Cornea. 2016 Dec;35(12):1662-1664.
- ↑ Knorz MC, Vossmerbaeumer U. Comparison of flap adhesion strength using the Amadeus microkeratome and the IntraLase iFS femtosecond laser in rabbits. J Refract Surg 2008;24:875–8.
- ↑ Holt DG, Sikder S, Mifflin MD. Surgical management of traumatic LASIK flap dislocation with macrostriae and epithelial ingrowth 14 years postoperatively. J Cataract Refract Surg 2012;38:357-61.
- ↑ Tetz M, Werner L, Müller M, Dietze U. Late traumatic LASIK flap loss during contact sport. J Cataract Refract Surg 2007;33:1332-5.
- ↑ Kim JS, Chung B, Lee T, et al. Management of long-standing partially torn and flipped laser in situ keratomileusis flaps. J Cataract Refract Surg 2015;41:464-7.
- ↑ Lyle WA, Jin GJ. Interface fluid associated with diffuse lamellar keratitis and epithelial ingrowth after laser in situ keratomileusis. J Cataract Refract Surg 1999;25:1009-12.
- ↑ Jump up to: 80.0 80.1 Kumar S. Traumatic interface oedema two years after LASIK. Clin Exp Optom 2009;92:395-6.
- ↑ Jump up to: 81.0 81.1 Liu X, Ling S, Gao X, et al. Pressure-induced stromal keratopathy as a result of ocular trauma after laser in situ keratomileusis. JAMA Ophthalmol 2013;131:1070-2.
- ↑ Jump up to: 82.0 82.1 Bushley DM, Holzinger KA, Winkle RK, et al. Lamellar interface fluid accumulation following traumatic corneal perforation and laser in situ keratomileusis. J Cataract Refract Surg 2005;31:1249-51.
- ↑ Jump up to: 83.0 83.1 Smith RJ, Maloney RK. Diffuse lamellar keratitis. A new syndrome in lamellar refractive surgery. Ophthalmology 1998;105:1721-6.
- ↑ Maddox R, Hatsis AP. Sands of Sahara syndrome interface inflammation with flap melt following LASIK. Ocular Surgery news. Thorofare, NJ. SLACK, Inc 1998;41-42.
- ↑ Cheng AC, Rao SK, Leung GY, et al. Late traumatic flap dislocations after LASIK. J Refract Surg 2006;22:500-4.
- ↑ Kim JS, Chung B, Lee T, et al. Management of long-standing partially torn and flipped laser in situ keratomileusis flaps. J Cataract Refract Surg 2015;41:464-7.
- ↑ Ursea R, Feng MT. Traumatic Flap Striae 6 years after LASIK: Case report and literature review. J Refract Surg 2010;26:899-905.
- ↑ Liu L,Song FZ, Bao LY. Histopathological study of corneal flap striae following laser in situ keratomileusis in rabbits. Exp Ther Med 2015;9:895-900.
- ↑ Karp KO, Hersh PS, Epstein RJ. Delayed keratitis after laser in situ keratomileusis. J Cataract Refract Surg 2000;26:925–8.
- ↑ Jump up to: 90.0 90.1 Karp CL, Tuli SS, Yoo SH, et al. Infectious keratitis after LASIK. Ophthalmology 2003;110:503-10.
- ↑ Read RW, Chuck RS, Rao NA, Smith RE. Traumatic Acremonium atrogriseum keratitis following laser-assisted in situ keratomileusis. Arch Ophthalmol 2000;118:418-21.
- ↑ Lee GA, Gray TB, Dart JK, et al. Acanthamoeba sclerokeratitis: treatment with systemic immunosuppression. Ophthalmology 2002:109:1178-82.
- ↑ Jump up to: 93.0 93.1 Reinstein DZ, Archer TJ, Randleman JB. Mathematical model to compare the relative tensile strength of the cornea after PRK, LASIK, and small incision lenticule extraction. J Refract Surg. 2013 Jul;29(7):454-60.
- ↑ Agca A, Demirok A, Cankaya K et al. Comparison of visual acuity and higher-order aberrations after femtosecond lenticule extraction and small-incision lenticule extraction. Cont Lens Anterior Eye 2014;37:292-6.
- ↑ Kanellopoulos AJ. Comparison of corneal biomechanics after myopic small-incision lenticule extraction compared to LASIK: an ex vivo study. Clin Ophthalmol. 2018 Jan 25;12:237-245.
- ↑ Jump up to: 96.0 96.1 Krueger RR, Meister CS. A review of small incision lenticule extraction complications. Curr Opin Ophthalmol. 2018 Jul;29(4):292-298.