Wide Field Retinal Imaging Systems
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
Introduction
Retinal imaging involves creating a two-dimensional image of the three-dimensional (3D) retinal tissue. Such retinal imaging techniques are indispensable for diagnosis and management of disease processes in ophthalmic practice. These help ophthalmic practitioners directly to view the retinal disease and plan treatment according to the pathology. Retinal imaging techniques are useful in diagnosis and management of ocular and systemic disorders like diabetic retinopathy, hypertensive retinopathy, age-related macular degeneration, vascular pathologies (vascular occlusions, vasculitis, etc), retinal detachments, glaucomas, systemic infections, leukemias, systemic malignancies with ocular metastasis, and others.
The Past in Retinal Imaging
Dating back to the early 20th century, ophthalmologists relied upon the tedious process of capturing fundus images and fundus fluorescein angiography (FFA) on film rolls. The late 20th century and earlier part of 21st century saw advancements in retinal imaging techniques. Since the advent of digital imaging, all traditional fundus cameras have been replaced by digital systems.
The most impactful advancement in the recent years in retinal imaging has been the introduction of Optical Coherence Tomography (OCT). Since its introduction in the 1990s, much has changed in our understanding of many retinochoroidal diseases, in our approach to management of common conditions and in our definitions of treatment success and end-point in a lot of those diseases.[1] OCT has been pivotal in identifying and understanding may diseases like Idiopathic macular hole and vitreomacular traction syndrome, myopic traction maculopathy, macular telangiectasia type 2, and pachychoroid spectrum of diseases. In many instances, OCT has obviated the need for repeated fluorescein angiography for continued management decisions. High speed OCT machines make it possible to acquire large number of A-scans and to convert them into highly resolved volume data. High speed OCT machines and some novel algorithms of OCT signal analysis has also led to the development of OCT angiography (OCT-A).
The Evolution in Retinal Imaging
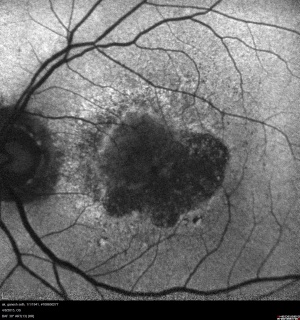
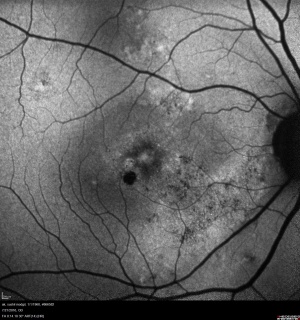
Retinal imaging techniques have evolved at a remarkable pace in the last two decades. Wide field imaging (WFI) and ultra wide field imaging (UWFI) are now increasingly popular. WFI refers to imaging beyond 50 degrees field area. UWFI systems can image upto 200 degrees as in Optos.[2] They are well capable of imaging over 80% of the retinal surface area. The peripheral retina can be photographed with small pupils[3][4] in instances where dilated peripheral fundus examination may be limited due to pupil size. Besides imaging, WFI also provides valuable information about the peripheral vasculature and other retinal lesions that might otherwise be missed with traditional imaging systems.
The modern systems allowing 100° to 200° view of fundus include:
- Pomerantzeff camera (Contact)[5]- Equator-plus camera (Film camera). This could image 148 degrees from the nodal point. This used a separate illumination source ('two illuminating fiber bundles, one for the central field and one for the periphery, at different locations on the cornea and different inclinations of incidence') from the camera. The limitations of this camera included absence of high resolution and brilliance/bright artefacts at the peripheral retina due to the peripheral illumination. The patient was imaged in sitting position.
- Retcam (Clarity Medical Systems, Inc., Pleasanton, CA, USA) (Contact)- maximum 130 degrees. The patient is usually supine, but images can be captured in adult or cooperative patients in sitting position also.
- Panoret-1000™ camera (Medibell Medical Vision Technologies, Haifa, Israel), (Contact)- 130 degrees. It had digital camera. It used transscleral illumination. Patient was imaged in supine position. The resolution was reasonable (20 microns) with minimal to no peripheral retinal brightness due to the illumination. It is currently no available commercially.
- Optos® camera (Optos PLC, Dunfermline, UK) (Noncontact)- Maximum 200 degrees. Patient is imaged in sitting position or in cases of babies in flying baby position. It gives pseudocolor images.
- Heidelberg Spectralis with the Staurenghi lens (Ocular Staurenghi 230 SLO Retina Lens; Ocular Instruments Inc, Bellevue, WA, USA) (Noncontact for 105 degree and contact for 150 degree Staurenghi lens)- Pseudocolor images.
- Clarus® 500 (Carl Zeiss Meditec) (Noncontact)- 133º in single image and 200º in two images - noncontact imaging - true color imaging and autofluorescence modes (blue, green and infrared)
Today, retinal imaging includes near histopathological examination of retina, viewing much beyond the equator, non-invasive ways of visualizing the retinal vasculature, health of Retinal Pigment Epithelium (RPE), photoreceptor density, quantifying various fundus pigments, the blood flow, oxygenation, the molecular transport and an ever increasing number of highly exciting things.[1]
Advantages of modern digital WFI and UWFI systems:[6]
- Enhanced resolution
- Shorter image processing time
- Faster image acquisition
- Ease of image duplication, manipulation and
- Possibility of image transmission via electronic route
- Better acquisition in eyes with cataract than a traditional fundus camera
- Non-compliant young pediatric patients to dilated retinal examination
- Patients with very small pupils
- Simultaneous imaging of central and peripheral retina; use of UWF FA to evaluate peripheral retinal ischemia in patients with diabetic macular edema and other vascular complications in the central macula
Confocal scanning laser ophthalmoscopy imaging (CSLO) systems
Advances in high-resolution OCT currently under development promise an even more detailed fundus representation. The integration of the confocal scanning laser ophthalmoscope (CSLO) and OCT has produced a dynamic new instrument, the OCT ophthalmoscope, which simultaneously images the fundus in numerous ways with point to point correlation.[6] CSLO systems use laser light to illuminate the retina, instead of bright flashes of light. This reduces scatter of light in images acquired.
Examples of cSLO-based ultrawidefield imaging (UWFI) systems include:
- the Optos® camera (Optos PLC, Dunfermline, UK),
- the Spectralis® (Heidelberg Retina Angiograph (HRA 2), Heidelberg Engineering, Germany).
Multimodal Imaging with WFI and UFWI systems
A great advantage offered by many of the present WFI and UWFI systems is the possibility of simultaneous acquisition of fundus fluorescein agniography (FFA), indocyanine angiography (ICGA), red-free photography, fundus photography, color fundus stereo imaging, adaptics optics CSLO, hyperspectral retinal imaging, fundus autofluorescence (FAF); including blue-reflectance (BAF), infrared reflectance (IRAF) or green reflectance (GAF).
| Available Platforms/Devices | Type of lens system | Principle | Field of view | Facilities available | ||||
|---|---|---|---|---|---|---|---|---|
| WFI | Heidelberg Spectralis | Non-contact | SD-OCT with CSLO | 55° (upto 105° with HRA 2) | FFA, ICGA, FAF (BAF and IRAF) | |||
| Contact | SD-COT with CSLO using Staurenghi Lens | 150° | FFA, ICGA, FAF (BAF and IRAF) | |||||
| RetCam 3 | Contact | Optical light source to obtain high resolution | Field depends on lens used among the five changeable lens systems[7]:
-130° ( pediatric retina and adult anterior chamber), -120°( pediatric and young adult), -80°(high contrast pediatric and adult), -30° (high magnification) and Portrait (external imaging) |
FFA, ICGA | ||||
| Clarus 500 | Noncontact | Slit Scanning Ophthalmoscope also referred to as Broad Line Fundus Imaging (BLFI) |
|
FAF (BAF, GAF and IRAF) | ||||
| UWFI | Optos Optomap | Noncontact | CSLO-based | 200 | FFA, FAF (GAF, IRAF) |
Applications of WFI and UWFI in Ocular Conditions
- Retinal Vascular Occlusions
- Retinal Vasculitis
- Pediatric Retinal Disorders
- Posterior Uveitis - infectious and non-infectious
- Peripheral Retinal Detachments
- Peripheral Polypoidal Choroidal Vasculopathy (Peripheral PCV)
- Peripheral Retinal Degenerations
- Peripheral retinoschisis
- Familial Exudative Vitreoretinopathy (FEVR)
- Eales' disease
- Acute Retinal Necrosis[8]
- Retinal detachments - exudative and tractional
- Peripheral retinal lesions which predispose to retinal detachment
- Screening for diabetic retinopathy
- Scenerios when scleral depression may be contraindicated
- Ocular tumors
Limitations of WFI and UWFI
- Difficulty to precisely measure the retinal surface area in order to estimation size and dimensions of retinal lesions.
- Image artifacts
- Conversion of a 3D surface to 2D image is still a challenge in retinal imaging
The Future Direction
Advanced imaging techniques have revolutionized the fundus examination in ophthalmology. Future in retinal imaging is expected to be as exciting as the recent journey has been, if not more. Research prototype swept source OCT ( multi-MHz Fourier domain mode-locked /FDML OCT) with speeds as high as 6700000 A scans/sec have been reported in recent literature.[1] [9]What this can do to the quality, speed and the quantity of data that can be acquired is something everyone is waiting to witness. With such high speed OCTs, 4-D intraoperative OCT may not be very distant. Recently, a frequency-swept light source called as the vertical cavity surface emitting laser was introduced with a very high imaging range of up to 50mm. It has been reported to image the entire eye, including anterior segment, lens, vitreous, retina, choroid and sclera in a single OCT – A 3D OCT image of the entire eye!
Ultra wide field indo cyanine green angiography is being looked forward to and is expected to provide more insights into diseases like CSC and polypoidal choroidal vasculopthy. Quantitative (AF) Auto Fluorescence is a way of quantifying the amount of AF emitted by the RPE. It has been shown that even normal looking and normal testing fundus in conditions like Stargardt disease show massive accumulation of autofluorescent material providing us new insight into disease and a possible means of prognosticating the patient. Adaptive Optics-Scanning Laser Ophthalmoscopes with fluorescein angiography filters are expected which will provide histopathological resolution of macular vascular architecture and may change the way we manage macular ischemia.
Conclusion
As advancements continue to develop in the field of retinal imaging, our understanding of ocular disease processes continues to improve. Newer technologies, which address the need of ophthalmologists towards achieving the proper diagnosis and appropriate management of disease entitities, have helped improve the patient care and management ultimately in our current ophthalmic practice.
References
- ↑ 1.0 1.1 1.2 Atul Kumar. Retinal Imaging-Present and future. Off Sci J Delhi Ophthalmol Soc. 2017;27(4):241-242. doi:10.7869/djo.257.
- ↑ Witmer MT, Kiss S. Wide-field imaging of the retina. Surv Ophthalmol. 2013 Mar-Apr;58(2):143-54. doi: 10.1016/j.survophthal.2012.07.003. Epub 2013 Jan 29. Review. PubMed PMID: 23369515.
- ↑ Tripathy K, Chawla R, Venkatesh P, Sharma YR, Vohra R. Ultrawide Field Imaging in Uveitic Non-dilating Pupils. J Ophthalmic Vis Res. 2017 Apr-Jun;12(2):232-233. doi: 10.4103/2008-322X.205360. PubMed PMID: 28540019; PubMed Central PMCID: PMC5423381
- ↑ Tripathy K, Chawla R, Vohra R. Evaluation of the fundus in poorly dilating diabetic pupils using ultrawide field imaging. Clin Exp Optom. 2017 Nov;100(6):735-736. doi: 10.1111/cxo.12484. Epub 2016 Oct 5. PubMed PMID: 27704602.
- ↑ Pomerantzeff O. Equator-plus camera. Invest Ophthalmol. 1975 May;14(5):401-6. PubMed PMID: 1126828.
- ↑ 6.0 6.1 Yannuzzi LA, Ober MD, Slakter JS, Spaide RF, Fisher YL, Flower RW, Rosen R. Ophthalmic fundus imaging: today and beyond. AmJ Ophthalmol. 2004;137(3):511-524. doi:10.1016/j.ajo.2003.12.035.
- ↑ http://www.claritymsi.com/us/downloads/Lenses&Images_2009_ f.pdf as accessed on 4 May 2015.
- ↑ Tripathy K, Sharma YR, Gogia V, Venkatesh P, Singh SK, Vohra R. Serial ultra wide field imaging for following up acute retinal necrosis cases. Oman J Ophthalmol. 2015 Jan-Apr;8(1):71-2. doi: 10.4103/0974-620X.149896. PubMed PMID: 25709284; PubMed Central PMCID: PMC4333553.
- ↑ Klein T, Wieser W, Reznicek L, Neubauer A, Kampik A, Huber R. Multi-MHz retinal OCT. Biomed Opt Express. 2013;4(10):1890–1908. Published 2013 Aug 30. doi:10.1364/BOE.4.001890