Optical Coherence Tomography Angiography
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
Optical Coherence Tomography Angiography
Overview
Optical coherence tomography angiography (OCT-A) has emerged as a non-invasive technique for imaging the microvasculature of the retina and the choroid. The first clinical studies using this innovative technology were published in 2014 .[1]
OCT-A technology uses laser light reflectance of the surface of moving red blood cells to accurately depict vessels through different segmented areas of the eye, thus eliminating the need for intravascular dyes.[2] The OCT scan of a patient's retina consists of multiple individual A-scans, which when compiled into a B-scan provides cross-sectional structural information. With OCT-A technology, the same tissue area is repeatedly imaged and differences are analyzed between scans (over time), thus allowing one to detect zones containing high flow rates (i.e. with marked changes between scans) and zones with slower, or no flow at all, which will be similar among scans.[3]
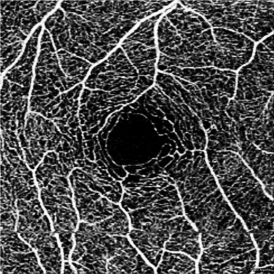
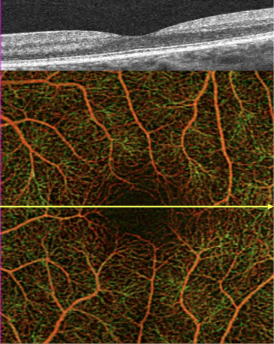
Light is emitted through either a spectral domain OCT (SD-OCT), with a wavelength of near 800nm; or a swept-source OCT (SS-OCT), which utilizes a longer wavelength, close to 1050nm. Longer wavelengths have a deeper tissue penetrance, but a slightly lower axial resolution. OCT-A employs two methods for motion detection: amplitude decorrelation or phase variance. The former detects differences in amplitude between two different OCT B-scans. Phase variance is related to the emitted light wave properties, and the variation of phase when it intercepts moving objects. To improve visualization and reduce background noise from normal small eye movements, two averaging methods - split spectrum amplitude decorrelation technique and volume averaging - were developed.[4][5] These OCT-A algorithms produce an image (3mm2 to 12mm2) that is segmented, by standard, into four zones: the superficial retinal plexus, the deep retinal plexus, the outer retina and the choriocapillaris. Applied to the optic disc it includes its full depth.[6][7]
Currently, there are currently 4 main commercially available OCT-A devices[8]:
- ZEISS Angioplex™ OCT angiographic imaging on the CIRRUS™ HD-OCT platform, with a scanning rate up to 68,000 A-scans per second and an improved tracking software known as FastTrac™. A three-dimensional image is obtained depicting erythrocyte flow as well as the microvasculature of the superficial, deep, and avascular layers of the retina.
- Optovue AngioVue® (Optovue, Inc., Freemont, CA), which uses split-spectrum amplitude-decorrelation angiography algorithm, which minimizes motion noise. This system also allows quantitative analysis, since it provides numerical data about flow area and flow density maps.
- Topcon® uses a different algorithm, OCTA RatioAnalysis, which benefits from being paired with SD-OCT, and improves detection sensitivity of low blood flow and reduced motion artifacts without compromising axial resolution.
- Heidelberg engineering® uses the active eye-tracking system (TruTrack™) that assesses simultaneously fundus and OCT images acquisition in order to achieve a better signal-to-noise ratio.
Advantages over conventional angiography methods
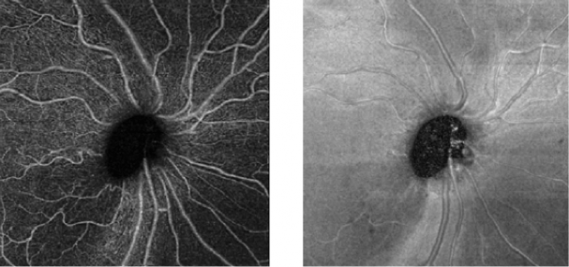
The main advantages are the shorter acquisition time and that it is a non-invasive process. Fluorescein and indocyanine-green angiography require an injectable dye (which takes time to reach retinal vessels, and may be associated with systemic adverse effects and even anaphylatic reactions[2]).
One asset of this OCT-based approach is that it provides a quantitative analysis of the retinal vessels (in addition to the qualitative analysis done on standard angiography). Moreover, and contrary to the "2-D" conventional angiograms, OCT-A technology provides "3-D" imaging information of the macula and visualizes peripapillary capillaries that supply the retinal nerve fiber layer.[6]
However, some limitations and artifacts are important to consider in the interpretation of OCT-A images (see section below).
Clinical Applications
Retina
As a fast, safe and noninvasive procedure to assess the chorioretinal microvasculature, OCT-A has been increasingly used in retinal diseases. The number of studies reporting new findings and utilities is exponentially growing.
Therefore, only a brief description of current uses is available in this article and further information should be looked in the available ophthalmology journals.
OCT-A has been reported to be useful in the diagnosis and understanding of many retinal conditions, namely:
- Diabetic retinopathy - identifying neovascular complexes, and quantifying foveal avascular zone and nonperfused areas, showing good agreement with FA findings. [9][10][11]
- Dry age-related macular degeneration - a general decrease in choriocapillaris flow has been reported, typically extending beyond the borders of areas of atrophy. Devices using SS-OCT are associated with better definition of choroidal vasculature changes.[12][13]
- Wet age-related macular degeneration - qualitative and quantitative analysis of choroidal neovascular membranes (CNVM), being able to classify them, and to follow-up structural changes after intravitreal injections. It has also been raised the potential for detection of these neovascular complexes in non-exudative cases, which would be difficult to detect using SD-OCT or FA, and thus contribute to a more effective and closer follow-up.[14][15]
- Central serous corioretinopathy - overlap between findings in FA of a pigmented epithelium detachment (PED) and CNVM may lead to situation of misdiagnosis. Especially in suspicious cases of flat and irregular PEDs, OCT-A may be helpful in diagnosis and management of CNVM.[16] Although some reports mention a decreased choriocapilaris blood flow[17][18], one should pay attention to signal strenght in en face images before interpreting this as hypoperfusion.
- Vascular occlusions - evaluation of nonperfused areas and the integrity of superficial and deep plexus. The preservation of the deep vasculature has been associated with better visual outcomes.[19][20]
- Macular telangiectasia - identification of the dilated, irregular telangiectatic vessels and, occasionally, the choroidal communication. Since OCT-A does not detect leakage, its combination with the OCT B-scan may help stage and manage MacTel without using FA.[21][22]
- Choroidal neovascular membranes of miscellaneous causes (e.g. associated with high myopia), with good sensitivity and specificity for detection.[23]
Glaucoma
OCT-A is also gaining increasing popularity for optic nerve disorders assessment, such as glaucoma.
It has been reported as a useful tool for evaluating optic disc perfusion in glaucomatous eyes, since attenuated peripapillary and macular vessel density was detectable in pre-perimetric glaucoma patients. Therefore, there is enthusiasm about the role of OCT-A in early detection of glaucomatous damage. Moreover, the quantitative data from these retinal vessels may prove useful in analysing metabolic activity from the inner layers of the retina and thus provide further advances in monitoring function and progression in this disease.[14][24]
It may also prove useful as a tool for ocular blood flow research and thus to help uncover non-IOP related mechanisms in this disease.
Uveitis
Francesco Pichi and collaborators recently reviewed the uses and importance of OCT-A in uveitis.[8] For the sake of this article, only a brief summary will be reported.
The authors divided the findings among the standard layers obtained with OCT-A:
- Superficial retinal capillary plexus - involved in inflammatory vasculitis, in which OCT-A is able to detect capillary dropout of superficial retinal vessels, capillary remodeling and a lower vessel density in uveitic eyes. The vascular reserve can be interpreted, which may be useful in management decisions. Also, in birdshot corioretinopathy, it has been suggested by recent OCT-A findings that the main ischemic insult responsible for secondary macular thinning may be in these inner retinal vessels.[25][26]
- Deep retinal capillary plexus - although smaller changes were detected when compared to superficial plexus in inflammatory conditions, OCT-A is able to detect patterns associated with cystoid macular edema. However, one should always bear in mind the potential for artifacts in deep plexus visualisation (see below).[25][27]
- Choriocapillaris - in inflammatory conditions associated with choroidal flow reduction or ischemia, OCT-A was able to detect flow void areas, that correlated with indocyanine-green angiography findings.[8]
The detection and evaluation of choroidal vascular membranes may be similarly achieved with OCT-A in uveitis, as in the other causes referred above.[28]Specifically in inflammatory conditions, OCT-A has the advantage of acquiring three-dimensional data, and potentially improving our understanding of the pathophysiology of these diseases as well as their follow-up and management. However, multimodal imaging is still the option of choice in the diagnosis and management of uveitis.[8]
Vascular Metrics
While clinicians primarily utilize OCT-A to qualitatively assess retinal microvasculature, researchers in recent years have aimed to develop more quantitative approaches.[29] These include the development of several vascular metrics that aim to quantify vascular features such as density and morphology. Though not exhaustive, below are several commonly used metrics.
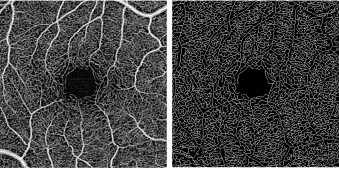
Vessel Area Density – a unitless measure that reflects the proportion of the OCT angiogram that is occupied by vessels of all caliber. This is typically accomplished by first binarizing the image such that the area occupied by vessels is comprised of white pixels and the avascular area is comprised of black pixels. A proportion of the white pixels divided by the total pixels in the image provides a measure of vessel density.
Vessel Skeletal Density – One limitation of vessel area density is the variability of vessel caliber among OCT angiograms in different eyes. For example, one eye may by chance have a greater number of larger vessels than another within a set window, thereby giving a false impression that it has increased density. Vessel skeletal density adjust for this variability by iteratively deleting outer pixels of each vessel such that each individual vessel, regardless of size, is represented by only a single line of pixels.
Vessel Diameter Index – this value reflects the average vessel diameter within an OCT angiogram and is calculated by dividing vessel area density by vessel skeletal density.
Artifacts
Media Opacities - media opacities such as corneal scarring, cataracts, posterior capsular opacification, and vitreous floaters may lead to signal attenuation and shadowing artifact.[3] These may obscure portions of the OCT angiogram or lead to diffuse reduction in image quality.
Projection Artifact - Projection artifact is inevitable and is related to light that traverses blood vessels and is reflected back by deeper layers (e.g. pigment retinal epithelium), which will appear in the final deep image with a similar vascular pattern as the overlying superficial vessels.[30]
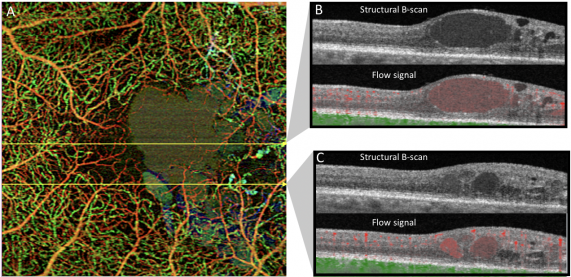
Segmentation Error - Automated segmentation of a structural abnormal retina is an unavoidable limitation of OCT-A imaging. In cases of pigment epithelial detachments (PED), one should look carefully for segmentation errors and manually edit layers if deemed necessary for a correct interpretation.[31]
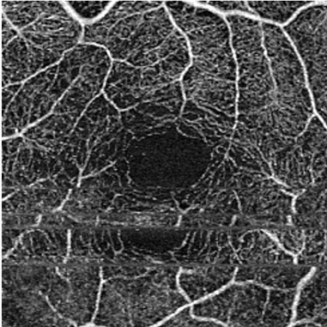
Motion Artifact - OCT-A examination is motion-sensitive and requires patients to be able to reasonably fixate, which may be difficult to obtain for the visually impaired.[11] Excessive motion of the eye can lead to motion artifacts as seen in the image. This can be overcome to some extent with a tracking feature present on most devices.
Extravascular signal from exudative edema - macular edema may appear as hypo- or hyper-reflective on structural OCTs. Hyper-reflective fluid, a form of exudate, is thought to be caused by suspended particles. OCT-A devices may capture the motion of these particles, leading to extravascular artifact. Transudative macular edema, in contrast, will not appear on OCT angiograms.
References
- ↑ Fingler J, Readhead C, Schwartz DM, Fraser SE. Phase-contrast OCT imaging of transverse flows in the mouse retina and choroid. Investig Ophthalmol Vis Sci. 2008;49(11):5055-5059.
- ↑ Jump up to: 2.0 2.1 Koustenis A, Harris A, Gross J, Januleviciene I, Shah A, Siesky B. Optical coherence tomography angiography: an overview of the technology and an assessment of applications for clinical research. Br J Ophthalmol. 2017 Jan;101(1): 16-20
- ↑ Jump up to: 3.0 3.1 Spaide RF, Klancnik JM, Cooney MJ. Retinal Vascular Layers Imaged by Fluorescein Angiography and Optical Coherence Tomography Angiography. JAMA Ophthalmol. 2015;133(1):45.
- ↑ Gorczynska I, Migacz J V, Zawadzki RJ, Capps AG, Werner JS. Comparison of amplitude-decorrelation, speckle-variance and phase-variance OCT angiography methods for imaging the human retina and choroid. Biomed Opt Express. 2016;7(3):911-942.
- ↑ Jia Y, Tan O, Tokayer J, et al. Split-spectrum amplitude-decorrelation angiography with optical coherence tomography. Opt Express. 2012;20(4):4710-4725.
- ↑ Jump up to: 6.0 6.1 Jia Y, Morrison JC, Tokayer J, et al. Quantitative OCT angiography of optic nerve head blood flow. Biomed Opt Express. 2012;3(12):3127-3137.
- ↑ Spaide RF, Curcio CA. Evaluation of Segmentation of the Superficial and Deep Vascular Layers of the Retina by Optical Coherence Tomography Angiography Instruments in Normal Eyes. JAMA Ophthalmol. 2017;135(3):259
- ↑ Jump up to: 8.0 8.1 8.2 8.3 Pichi F, Sarraf D, Arepalli S, et al. The application of optical coherence tomography angiography in uveitis and inflammatory eye diseases. Prog Retin Eye Res. 2017 Apr 29.
- ↑ Samara WA, Shahlaee A, Adam MK, et al. Quantification of Diabetic Macular Ischemia Using Optical Coherence Tomography Angiography and Its Relationship with Visual Acuity. Ophthalmology. 2017;124(2):235-244.
- ↑ Spaide RF. Volume-Rendered Optical Coherence Tomography of Diabetic Retinopathy Pilot Study. Am J Ophthalmol. 2015;160(6):1200-1210.
- ↑ Jump up to: 11.0 11.1 De Oliveira PRC, Berger AR, Chow DR. Optical coherence tomography angiography in chorioretinal disorders. Can J Ophthalmol / J Can d’Ophtalmologie. 2017;52(1):125-136.
- ↑ Waheed NK, Moult EM, Fujimoto JG, Rosenfeld PJ. Optical Coherence Tomography Angiography of Dry Age-Related Macular Degeneration. In: Developments in Ophthalmology.Vol 56.; 2016:91-100.
- ↑ Choi W, Moult EM, Waheed NK, et al. Ultrahigh-Speed, Swept-Source Optical Coherence Tomography Angiography in Nonexudative Age-Related Macular Degeneration with Geographic Atrophy. Ophthalmology. 2015;122(12):2532-2544.
- ↑ Jump up to: 14.0 14.1 de Carlo TE, Romano A, Waheed NK, Duker JS. A review of optical coherence tomography angiography (OCTA). Int J Retin Vitr. 2015;1(1):5.
- ↑ Huang D, Jia Y, Rispoli M, Tan O, Lumbroso B. Optical Coherence Tomography Angiography Of Time Course Of Choroidal Neovascularization In Response To Anti-Angiogenic Treatment. Retina. 2015;35(11):2260-2264.
- ↑ de Carlo TE, Rosenblatt A, Goldstein M, Baumal CR, Loewenstein A, Duker JS. Vascularization of Irregular Retinal Pigment Epithelial Detachments in Chronic Central Serous Chorioretinopathy Evaluated With OCT Angiography. Ophthalmic Surgery, Lasers Imaging Retin. 2016;47(2):128-133.
- ↑ Teussink MM, Breukink MB, van Grinsven MJJP, et al. OCT Angiography Compared to Fluorescein and Indocyanine Green Angiography in Chronic Central Serous Chorioretinopathy. Investig Opthalmology Vis Sci. 2015;56(9):5229.
- ↑ McClintic SM, Jia Y, Huang D, Bailey ST. Optical Coherence Tomographic Angiography of Choroidal Neovascularization Associated With Central Serous Chorioretinopathy. JAMA Ophthalmol. 2015;133(10):1212.
- ↑ Wakabayashi T, Sato T, Hara-Ueno C, et al. Retinal Microvasculature and Visual Acuity in Eyes With Branch Retinal Vein Occlusion: Imaging Analysis by Optical Coherence Tomography Angiography. Investig Opthalmology Vis Sci. 2017;58(4):2087.
- ↑ Kadomoto S, Muraoka Y, Ooto S, et al. Evaluation Of Macular Ischemia In Eyes With Branch Retinal Vein Occlusion. Retina. 2017:1.
- ↑ Zhang Q, Wang Rk, Chen C-L, Et Al. Swept Source Optical Coherence Tomography Angiography Of Neovascular Macular Telangiectasia Type 2. Retina. 2015;35(11):2285-2299.
- ↑ Spaide Rf, Suzuki M, Yannuzzi La, Matet A, Behar-Cohen F. Volume-Rendered Angiographic And Structural Optical Coherence Tomography Angiography Of Macular Telangiectasia Type 2. Retina. 2017;37(3):424-435.
- ↑ Querques L, Giuffrè C, Corvi F, et al. Optical coherence tomography angiography of myopic choroidal neovascularisation. Br J Ophthalmol. 2016:bjophthalmol-2016-309162.
- ↑ Yarmohammadi A, Zangwill LM, Diniz-Filho A, et al. Peripapillary and Macular Vessel Density in Patients with Glaucoma and Single-Hemifield Visual Field Defect. Ophthalmology. 2017;124(5):709-719.
- ↑ Jump up to: 25.0 25.1 Kim AY, Rodger DC, Shahidzadeh A, et al. Quantifying Retinal Microvascular Changes in Uveitis Using Spectral-Domain Optical Coherence Tomography Angiography. Am J Ophthalmol. 2016;171:101-112.
- ↑ Park JJ, Soetikno Bt, Fawzi Aa. Characterization Of The Middle Capillary Plexus Using Optical Coherence Tomography Angiography In Healthy And Diabetic Eyes. Retina. 2016;36(11):2039-2050.
- ↑ Phasukkijwatana N, Iafe N, Sarraf D. Optical Coherence Tomography Angiography Of A29 Birdshot Chorioretinopathy Complicated By Retinal Neovascularization. Retin Cases Brief Rep. 2017;11 Suppl 1:S68-S72.
- ↑ Levison AL, Baynes KM, Lowder CY, Kaiser PK, Srivastava SK. Choroidal neovascularisation on optical coherence tomography angiography in punctate inner choroidopathy and multifocal choroiditis. Br J Ophthalmol. 2017;101(5):616-622.
- ↑ Kim AY, Chu Z, Shahidzadeh A, Wang RK, Puliafito CA, Kashani AH. Quantifying microvascular density and morphology in diabetic retinopathy using spectral-domain optical coherence tomography angiography. Invest Ophthalmol Vis Sci. 2016;57:OCT362–OCT370.
- ↑ Spaide Rf, Fujimoto Jg, Waheed Nk. Image Artifacts In Optical Coherence Tomography Angiography. Retina. 2015;35(11):2163-2180.
- ↑ Chen FK, Viljoen RD, Bukowska DM. Classification of image artefacts in optical coherence tomography angiography of the choroid in macular diseases. Clin Experiment Ophthalmol. 2016;44(5):388-399.