Von Hippel-Lindau Syndrome
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
Disease Entity
Von Hippel-Lindau Syndrome (VHL)
A short video from AAO experts can be accessed here: https://www.aao.org/1-minute-video/von-hippel-lindau-disease-7
Disease
Von Hippel-Lindau Syndrome (VHL) is a rare, autosomal dominant, familial neoplastic disease that affects the central nervous system and multiple organs such as the kidneys, pancreas, adrenals, and reproductive organs. Mutations in the tumor suppressor gene VHL cause the disease, which commonly manifests as a variety of tumors such as hemangioblastomas of the retina and brain as well as renal cell carcinoma. The disease affects one in every 36,000 live births and shows near- complete penetrance (90%) by 65 years of age[1] [2]. Cerebellar lesions, which present around age 30, are one of the most common manifestations of VHL[3]. Diagnosis is confirmed with a positive familial history and at least one VHL-related tumor[3]. However, 20% of mutations are de novo, and diagnosis for patients with a negative family history is confirmed with the occurrence of two VHL-related tumors and at least one retinal hemangioblastoma[3]. Retinal hemangioblastomas present the earliest around age 20, but are not present in every diagnosed case of VHL[4].
VHL is clinically classified as:
Epidemiology
Von Hippel-Lindau disease manifests in the third to fourth decades of life, depending on the location of the tumors. The mean age of onset of retinal hemangioblastoma, one of the first manifestations, is around 26 years[1]. However, 5% of patients with retinal hemangioblastoma can present under the age of 10[4].
Pathophysiology
Von Hippel-Lindau disease can be split into two subtypes, Type 1 and Type 2, depending on the presence of pheochromocytomas[5]. Type 1 VHL has a low risk of pheochromocytomas, but both subtypes present with multiple organ tumors[5]. Retinal hemangioblastoma usually presents bilaterally and around the optic disc.
The VHL gene is located on chromosome 3p35 and encodes the pVHL protein[6][4]. One proposed mechanism of tumorigenesis for VHL involves the regulatory effect pVHL has on hypoxia-inducible factors (HIF). Under normal conditions, von Hippel-Lindau tumor suppressor pVHL ubiquitinates HIF for degradation. However, a lack of pVHL such as in VHL disease or hypoxia leads to stabilization of HIF-α and increased expression of tumorigenic molecules such as vascular endothelial growth factor (VEGF), platelet- derived growth factor peptide (PDGF), and transforming growth factor (TGF-a)[4]. Recently, more genes have been found to be involved in VHL-pheochromocytoma including Syndecan Binding Protein (SDCBP), Connective Tissue Growth Factor (CTGF), S, Cysteine Rich Protein 61 (CYR61/CCN1), Serpin Family E Member 1 (SERPINE1), Collagen Type III Alpha 1 Chain (COL3A1), Collagen Type I Alpha 1 Chain (COL1A1) and Collagen Type V Alpha 2 Chain (COL5A2)[7]
Diagnosis
Definitive diagnosis of Von Hippel-Lindau disease occurs in one of two ways. A diagnosis of familial VHL requires a positive family history of disease in addition to one VHL-associated tumor[3]. Those without a family history require two or more VHL-associated tumors for diagnosis[3]. Southern blots can be used on peripheral blood leukocytes to detect one of the more than 137 mutations in the VHL gene[4]. However, this method is less effective in those with genetic mosaicism[4]. Diagnosis of VHL-associated tumors is more varied.
Retinal hemangioblastoma is best diagnosed with pharmacological dilation of pupil and examination using an ophthalmoscope[4].
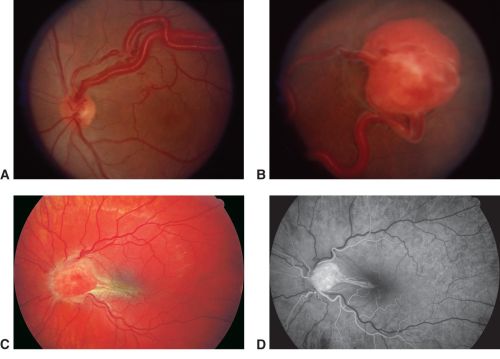
Retinal hemangioblastoma, fundus photo A-C, Fluorescein Angiography D. A. Observe the tortuous and dilated artery (feeder) and vein exiting through optic nerve head (draining), as well as the protruding retinal tumor with orange-yellow color present in the B. peripheral and C. optic nerve, with macular traction. D. Because of the arterial origin, this vascular lesion would be hyperfluorescent and bright in early phases of FA.
Both computed tomography (CT) and magnetic resonance imaging (MRI) will reveal large tumors expanding into the temporal bones for endolymphatic sac tumors (ELST)[8]. Abdominal CT reveals the size and quantity of renal lesions in renal cell carcinoma (RCC)[8]. Laboratory studies such as plasma free metanephrines can be used to detect pheochromocytoma[8].
Histopathological examination of retinal hemangioblastomas reveals the same pathology as central nervous system (CNS) hemangioblastomas: polygonal stromal cells surrounded by several vascular structures[8].
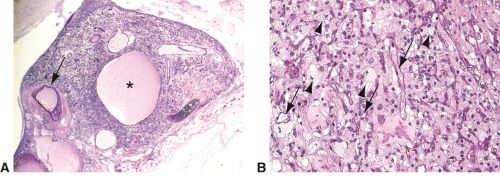
Retinal hemangioblastoma histology, PAS stains. Low power image-A. This retinal tumor shows high vessel density with thick walls (arrow) and cyst containing proteinaceous material (asterisk) High power image-B. The stromal cells are foamy and vacuolated (arrowheads) with many small channels resembling capillaries (arrows). VHL mutation is expressed in the stromal cells(arrowheads).
History and physical examination
Systemically, VHL disease most commonly presents with cerebellar hemangioblastomas, but manifestations can present on multiple parts of the body[9]. A physical examination involves checking for the possible consequences of the various tumors. Retinal hemangioblastomas specifically may be seen as a loss of visual activity upon eye examination due to the growth of lesion, which can cause exudation, hemorrhage, and serous/exudative retinal detachments[5]. As mentioned above, tumors may be seen with an ophthalmoscope.
CNS symptoms may be seen on patients with CNS hemangioblastomas, such as headache, vomiting, and ataxia[5]. Endolymphatic sac tumors may cause uni- or bilateral hearing loss[5]. Hearing loss may be caused by the tumor invading structures such as the cochlea and semicircular canals[10].Renal cell carcinoma may be felt as a solid mass upon palpation that presents with flank pain or hematuria[8]. Pheochromocytomas can cause hypertension, tachycardia, and sweating[5].
Differential diagnosis
The differential diagnosis of VHL contains the various VHL-associated tumors seen in isolation[5]. The disease is associated with numerous tumors, so a differential diagnosis would include isolated retinal hemangioblastoma, renal cell carcinoma, or CNS hemangioblastoma. Patients presenting with pheochromocytoma and no history of VHL may be presenting with either multiple endocrine neoplasia type 2, hereditary paraganglioma-pheochromocytoma syndrome, or neurofibromatosis 1[5]. These diseases are caused by a mutation in a gene separate from VHL. Menière disease is another disease that presents similarly to endolymphatic sac tumors[5]. Renal cell carcinoma may be caused by hereditary leiomyomatosis and renal cell cancer[5].
Screening and Surveillance
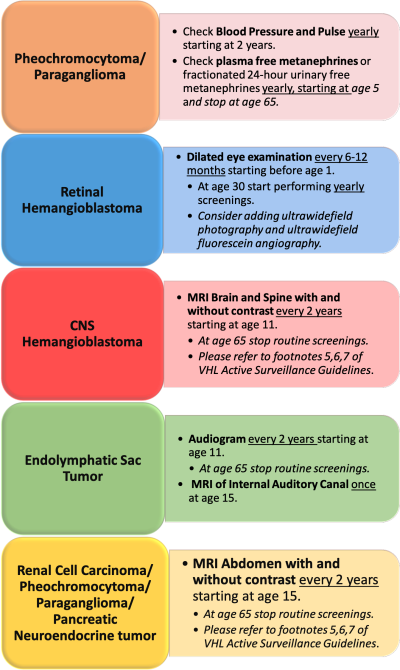
The mainstay to reduce morbidity and mortality is early identification of disease and progression of it by screenings and genetic testing. Given the genetic pattern of inheritance, a person is deemed “high risk” when a biological parent has VHL. The VHL Alliance has put forth a set of surveillance guidelines for those at high risk and those that test positive for VHL. [11]. These routine screenings can help identify the disease at an earlier age and mitigate the onset of worsening symptoms.
The following is an adapted graphic of the VHL Alliance Active Screening Guidelines:
Management
No guidelines exist for the management of VHL associated tumors, but various surgical and non-surgical interventions have been used previously. The location, size, and degree of anatomical involvement are key elements that determine how the different neoplasms are managed. Different lesions contain different time periods for screening, with biannual MRI scans for visceral lesions starting at age 16[5]. No guidelines exist for the management of VHL associated tumors, but various surgical and non-surgical interventions have been used previously.
Management
Various surgical and non-surgical interventions have been used previously. The location, size, and degree of anatomical involvement are key elements that determine how the different neoplasms are managed. Most interventions focus on shrinking or removing the tumors present in VHL disease.
CNS hemangioblastomas are standardly treated with surgical resection when they become symptomatic due to size [12]. In instances where the neoplasm is deemed inoperable, a technique that is increasing in popularity is stereotactic radiosurgery[8]. More recently FDA approved belzutifan, an HIF-2α inhibitor, has been used to treat neuro-associated tumors with some reports stating a 30% response in hemangioblastomas and almost half of them reducing the tumor size[13] [14][15]
Retinal hemangioblastomas treatment is needed if it compromises vision, treatment options involve diathermy and cryocoagulation to reduce the size of retinal angiomas[5]. Laser photocoagulation has shown efficacy when tumors are <1.5mm[16]. External beam radiotherapy may be used for larger tumors (diameter >4mm) which tend to be resistant against standard treatment [8]. Tumors found near the optic disc may be treated with intravitreal anti-VEGF therapy if necessary[8].
The following is a video that implements some of these techniques as well as excision. https://www.aao.org/clinical-video/retinal-capillary-hemangioblastoma-excision
Kidney involvement can be benign (cysts) or malignant (Renal Cell Carcinoma). Renal cell carcinomas that are 3 cm or more in diameter can be removed with a partial nephrectomy to prevent metastasis and maintain normal kidney function[4]. Smaller tumors could be surgically resected or undergo radiofrequency ablation or cryoablation. Pazopanib may be used to treat advanced renal cell carcinoma and is an FDA-approved treatment[5]
Pheochromocytomas surgically managed with an adrenalectomy can prevent some of the life-threatening complications present with pheochromocytomas such as hypertension and myocardial infarction.
Endolymphatic sac tumors could be considered for resection to prevent sensorineural hearing loss and possible vestibular symptoms[8]. Surgical resection with microsurgical techniques has proved to be an efficacious alternative. [17].
Prognosis
Despite no known cure, comprehensive multidisciplinary care that implements routine screenings has the potential to discover multi-systemic involvement early and decrease the secondary symptoms associated with these neoplasms. Renal cell carcinoma is the leading cause of mortality, and nephron-sparing surgeries can have a 10-year cancer survival rate of 81%[4][8]. However, even with these treatments, the life expectancy of VHL patients is 59.4 years for males and 48.4 years for females[8].
Survelliance
Recommend relatives to obtain genetic status. Some experts suggest patients to get yearly exams for neurologic, vision and hearing problems as well as monitor fo blood pressure and urine metanephrines.
Imaging may be done every 2 years including MRI brain and total spine (older than11 years-old) while MRI of abdomen may start at 15 years of age. MRI of the internal auditory canal may be suggested for asymptomatic patients older than 15 years of age.
Conclusion
Von Hippel-Lindau disease is a rare, autosomal dominant, familial neoplastic disease that is thought to arise due to the stabilization of HIF-α and its subsequent effects on tumorigenesis. This disease commonly presents with multiple-organ tumors that each cause symptoms ranging from hearing loss to visual impairment. Consequently, ophthalmologists can play a critical role in the diagnosis and treatment of this disease as one of the early symptoms is vision change due to exudation, hemorrhage, and serous retinal detachment[18].
Additional Resources
VHL national website.
References
- ↑ 1.0 1.1 Benjamin C, Yates JRW, Moore AT, et al. Clinical Features and Natural History of von Hippel-Lindau Disease. Qjm. 2012;77(2):1151-1163. doi:10.1093/qjmed/77.2.1151
- ↑ Ecol A, Desjardins RL, Latif F, et al. Identification of the von Hippel-Lindau Disease Tumor Suppressor Gene. Science (80). 1993;260:1317-1320.
- ↑ 3.0 3.1 3.2 3.3 3.4 Coppin L, Plouvier P, Crépin M, Jourdain A-S, Yahya EA, Richard S, Paillerets BB-d, Cardot-Bauters C, Lejeune S, Leclerc J, Pigny P, Optimization of next-generation sequencing technologies for von Hippel Lindau (VHL) mosaic mutation detection and development of confirmation methods, The Journal of Molecular Diagnostics (2019), doi: https://doi.org/10.1016/j.jmoldx.2019.01.005.
- ↑ 4.0 4.1 4.2 4.3 4.4 4.5 4.6 4.7 4.8 Lonser RR, Glenn GM, Walther M, et al. von Hippel-Lindau disease. Lancet. 2003;361:2059-2067.
- ↑ 5.00 5.01 5.02 5.03 5.04 5.05 5.06 5.07 5.08 5.09 5.10 5.11 5.12 van Leeuwaarde RS, Ahmad S, Links TP, et al. Von Hippel-Lindau Syndrome. 2000 May 17 [Updated 2018 Sep 6]. In: Adam MP, Ardinger HH, Pagon RA, et al., editors. GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle; 1993-2019. Available from: https://www.ncbi.nlm.nih.gov/books/NBK1463/
- ↑ Chen X, Xu G, Bi Q, Huang Y, Shao H, Jin M, Mesfin A, Zhang J, Cauda Equina Syndrome as the first Manifestation of von Hippel-Lindau Disease: A Case Report, World Neurosurgery (2019), doi: https://doi.org/10.1016/j.wneu.2019.01.269.
- ↑ Gao S, Liu L, Li Z, Pang Y, Shi J, Zhu F. Seven Novel Genes Related to Cell Proliferation and Migration of VHL-Mutated Pheochromocytoma. Front Endocrinol (Lausanne). 2021;12:598656. Published 2021 Mar 22. doi:10.3389/fendo.2021.598656
- ↑ 8.00 8.01 8.02 8.03 8.04 8.05 8.06 8.07 8.08 8.09 8.10 Varshney, Neha et al. “A Review of Von Hippel-Lindau Syndrome.” Journal of kidney cancer and VHL. 4.3 (2017): 20–29. Web.
- ↑ Poulsen MLM, Budtz-Jørgensen E, Bisgaard ML. Surveillance in von Hippel-Lindau disease (vHL). Clin Genet. 2010;77(1):49-59. doi:10.1111/j.1399-0004.2009.01281.x
- ↑ Oldfield EH, Kim HJ, Lonser RR, Vortmeyer AO, Choo DI, Butman JA. Tumors of the Endolymphatic Sac in von Hippel–Lindau Disease. N Engl J Med. 2004;350(24):2481-2486. doi:10.1056/nejmoa040666
- ↑ VHL Alliance. VHLA Suggested Active Surveillance Guidelines. VHL Alliance. https://www.vhl.org/suggested-active-screening-guidelines/. Accessed May 16, 2020.
- ↑ Jagannathan J, Lonser RR, Smith R, DeVroom HL, Oldfield EH. Surgical management of cerebellar hemangioblastomas in patients with von Hippel-Lindau disease. J Neurosurg. 2008;108(2):210‐222. doi:10.3171/JNS/2008/108/2/0210
- ↑ Ungprasert P, Sukpornchairak P, Moss BP, Ribeiro Neto ML, Culver DA. Neurosarcoidosis: an update on diagnosis and therapy. Expert Rev Neurother. 2022;22(8):695-705. doi:10.1080/14737175.2022.2108705
- ↑ Zhang Y, Nguyen CC, Zhang NT, et al. Neurological applications of belzutifan in von Hippel-Lindau disease. Neuro Oncol. 2023;25(5):827-838. doi:10.1093/neuonc/noac234
- ↑ van Leeuwaarde RS, Ahmad S, van Nesselrooij B, et al. Von Hippel-Lindau Syndrome. 2000 May 17 [Updated 2024 Feb 29]. In: Adam MP, Feldman J, Mirzaa GM, et al., editors. GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle; 1993-2024. Available from: https://www.ncbi.nlm.nih.gov/books/NBK1463/
- ↑ Singh AD, Nouri M, Shields CL, Shields JA, Perez N. Treatment of retinal capillary hemangioma. Ophthalmology. 2002;109(10):1799‐1806. doi:10.1016/s0161-6420(02)01177-6
- ↑ Friedman RA, Hoa M, Brackmann DE. Surgical management of endolymphatic sac tumors. J Neurol Surg B Skull Base. 2013;74(1):12‐19. doi:10.1055/s-0032-1329622
- ↑ Kreusel KM, Bechrakis NE, Krause L, Neumann HPH, Foerster MH. Retinal Angiomatosis in von Hippel-Lindau Disease. A Longitudinal Ophthalmologic Study. Ophthalmology. 2006;113(8):1418-1424. doi:10.1016/j.ophtha.2006.02.059