Viral Vectors for Gene Therapy
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
Viral Vectors for Gene Therapy
Background
Viral gene therapy has gained FDA approval for very few organs in the human body, and the eye is among them. The discovery of over 270 gene mutations responsible for inherited retinal diseases has sparked increased research into viral gene therapy as a potential treatment approach[1]. The majority of inherited retinal diseases are characterized by monogenic traits, with mutations manifesting in either photoreceptor cells or the retinal pigment epithelium (RPE). The transduction of the photoreceptor layer can vary based on the serotypes of the viral vectors and the method of entry[1].
In December 2017, the US Food and Drug Administration (FDA) approved the first gene therapy product, voretigene neparvovec (Luxturna) developed by Spark Therapeutics[2]. This was approved for treatment of pediatric patients with Leber Congenital Amaurosis, resulting from RPE65 gene deficiency[3]. The objective of viral gene therapy in ocular applications is to address retinal diseases with the aim of restoring functional vision and slowing the progression of the condition.
Benefits
The eye’s anatomy, accessibility, and immune-privilege collectively make it a favorable location for viral gene therapy. Being immune-privileged implies that the eye limits its inflammatory response in order to ensure preservation of vision even in the presence of swelling. The eye contains immunomodulatory molecules that act as strong inhibitors of inflammatory cells. In addition to the reduced immune response and inflammation toward introduced genetic material, the presence of a tight blood-retinal barrier further limits systemic drug exposure and subsequent immune and adverse reactions. Moreover, the retina is composed of terminally differentiated cells. This essentially lowers the risk of gene integration and chromosomal rearrangements, decreasing the risk of adverse events and complications.
Mechanism
Viral vectors have the ability to transport therapeutic genes into the nuclei of specific target cells. The technique utilizes the inherent capability of viruses to infect human genomes. Undesirable viral genetic sequences are substituted with the intended therapeutic genes, and subsequent infection of target cells with the modified viruses results in the integration of the therapeutic material into the cell nuclei. The goal is to augment and repair dysfunctional inherited genes. Specific drugs can be generated and introduced into the target cells, allowing for generation of the required drugs in vivo and ultimate transformation of the tissues into continuous bio-factories producing medications[4].
Types of Viral Vectors
There are currently three types of viral vector considerations for gene delivery that have been studied in preclinical and clinical trials, with varying uses based on their characteristics, effectiveness, and complications. The three vectors include adeno-associated virus (AAV), adenovirus (AV), and lentivirus.
Adeno-associated virus (AAV)
Adeno-associated virus (AAV) is the most commonly used vector at this time. It is a non-enveloped single stranded DNA virus belonging to the Parvoviridae family. There are currently 13 distinct serotypes confirmed that vary in capsid components[4]. Because of their tissue-specific tropisms and gene transfer efficacy and stability, AAV2, AAV4, AAV5, and AAV8 are the most commonly used adeno-associated virus (AAV) serotypes used as vectors[4][5]. AAV2 and AAV8 are capable of infecting retinal cells successfully from the vitreous, although they are limited to the inner retina, as reported by Bennet et al., [4], [5]. Subretinal delivery of AAV2 and AAV8 has demonstrated effective transduction of the retinal pigment epithelium (RPE) and photoreceptor layer respectively. Interestingly, the ability of AAV to transduce the retinal pigment epithelium versus photoreceptor cells depends on the serotype being injected into the subretinal space. AAV1, AAV4, and AAV6 are more specific to the RPE, while AAV5 and AAV8 are more specific to photoreceptor cells[6].
Among the general population, approximately 70% possess preexisting antibodies against AAV2, while 38% contain antibodies against AAV8. The presence of these preexisting antiviral antibodies has been linked to diminished gene expression after gene therapy. Consequently, AAV8-based vector therapy holds the potential for greater effectiveness than that of AAV2[4]. Although AAV has proven to be safe and effective along with a vast variety of clinical applications in a range of diseases, it has limited capacity to carry large genes, with a packaging capacity of about 4.8 kb.[4] [7],[8].
Therefore, this viral vector has restricted ability to code conditions encoded by larger genes, such as Usher syndrome and Stargardt disease. Nonetheless, its mild initiation of both an innate and adaptive immune response allows for stable long-term expression and thus makes it a favorable vector for a range of chronic ocular diseases. At present, over 30 clinical trials for ocular gene replacement are underway employing novel AAV vectors[6].
Adenovirus (AV)
Adenovirus is a non-enveloped double-stranded DNA virus. There is currently only a singular clinical trial, dedicated to the study of retinoblastoma, using adenovirus as the vector. This was the first vector to be studied, yet it is not currently utilized in any other clinical trials or treatments due to its strong immune response resulting in severe adverse reactions, such as fever, liver damage, systemic adenoviral infections, and death[4]. Moreover, this triggered immune response leads to limited transgene expression. It exhibits a wide spectrum of tissue tropism, allowing the transduction of both retina and the anterior segment. Another advantage of this vector is its capacity to carry large genes, up to 37 kb [8].
Lentivirus
Lentivirus is a complex, single-stranded RNA retrovirus that originates from primate lentiviruses, including the human immunodeficiency virus (HIV), equine infectious anemia virus (EIAV), and simian immunodeficiency virus[4][8]. Lentivirus successfully tranduses retinal pigment epithelium (RPE) cells, yet it does not effectively target photoreceptor cells. Unlike both adenovirus (AV) and adeno-associated virus (AAV) which are episomal and thus have low risk for insertional mutagenesis, lentivirus integrates its complementary DNA into the chromosome of target cells [9]. Consequently, this increases the potential of insertional mutagenesis[4][8]. This vector has the capacity to carry genes of 9-10 kb, smaller than that of the adenovirus, but larger than that of the adeno-associated virus[8].
Ocular Disease Application
Viral gene therapy has surfaced as a revolutionary platform, offering novel treatment possibilities for various ocular diseases, and its full potential is only starting to be realized. Using viral vectors to treat a variety of ocular conditions with underlying genetic factors is still a therapeutic approach in the early phases of development. Nonetheless, knowledge of the genetic aspects associated with inherited retinal diseases has significantly expanded with identification of substantial disease-causing genes, specifically within the last twenty years[6]. This has opened the doors for a wide range of preclinical and clinical studies utilizing the developing field of viral gene therapy as a therapeutic approach to a multitude of ocular conditions.
Inherited Retinal Diseases
With the advances in DNA sequencing, there have been approximately 300 genes identified to be associated with inherited retinal diseases, which has allowed opportunity for exploration of viral gene therapy as a therapeutic approach in the following conditions[8].
Leber Congenital Amaurosis (LCA)
Leber Congenital Amaurosis is a pediatric ocular condition resulting from a RPE65 gene mutation, a highly expressed gene in retinal pigment epithelium cells. This was the first ocular condition to receive FDA approval for the use of viral gene therapy as treatment. Phase III clinical trials of Luxturna (voretigene neparvovec) using AAV2 as the viral gene vector injected into the subretinal space showed both efficacy and safety [10]. This clinical trial involved 29 pediatric patients, less than 3 years old, who had the condition and a visual acuity of 20/60 or less. Ultimately, the results of the trials showed improvement of functional vision without any major complications, other than mild inflammation in two patients[4].
X-linked Retinoschisis (XLRS)
X-linked Retinoschisis is a juvenile retinal disorder resulting from a RS1 gene mutation, a gene that encodes retinoschisin which is a protein involved in organization of retinal cells. There are currently two clinical trials studying the use of AAV vectors expressing RS1 gene delivered intravitreally. Limitations in both studies include dose-dependent ocular inflammation[4].
Stargardt Disease
Stargardt Disease is a pediatric macular dystrophy resulting from a ABCA4 gene mutation, resulting in accumulation of lipofuscin pigment inside the retinal pigment epithelium. A phase I/II clinical trial studied the use of subretinal injection of lentivirus encoding the ABCA4 gene carrying equine infectious anemia viral vector[10]. Results showed a reduction of pigment accumulation inside of the retinal pigment epithelium[10]. Limitations of this using viral gene therapy for this condition include the large size of the ABCA4 gene. Lentiviral vectors prove to be the most fitting for ABCA4 gene transfer. The use of AAV2 as a vector for therapy is another possibility that requires further evaluation in this condition.
Choroideremia
Choroideremia is a chorioretinal dystrophy marked by gradual degeneration of the retinal pigment epithelium and photoreceptors resulting from a CHM gene mutation. There are currently nine clinical trials studying the use of AAV2 as a vector encoding REP1 for gene therapy via subretinal injection, except for one trial evaluating intravitreal injection[4]. There are a few challenges that have limited viral gene therapy research in this condition. There is a lack of animal model resemblance to the disease features, along with uncertainty regarding the retinal layer predominantly impacted during disease progression[4].
Usher Syndrome
Usher syndrome is marked by progressive vision and hearing loss resulting from a variation of MYO7A, USH2A, or GPR98 gene mutations. Due to the large size of the genetic material, lentiviral vectors prove to be the most fitting for gene transfer. There has been one terminated clinical trial that studied the use of subretinal injection of lentivirus encoding MYO7A. There is currently a phase I/II clinical trial studying intravitreal injection of RNA antisense oligonucleotide[4]. There is room for further extensive research addressing viral gene therapy in Usher Syndrome.
Achromatopsia
Achromatopsia is marked by retinal degeneration and dysfunctional cone photoreceptors resulting from 6 gene mutations – CNGA3, CNGB3, PDE6C, PDE6H, ATF6. There are ongoing phase I/II clinical trials studying subretinal injection of AAV viral vector encoding CNGA3 and CNGB3[4].2
Age-Related Macular Degeneration (AMD)
Age-Related Macular Degeneration is a multifaceted condition influenced by a combination of genetic and environmental factors that contribute to the development and progression of the disease. Studies have connected single-nucleotide polymorphisms (SNPs) with disease severity, and these genes encode components of the complement cascade.12 Subretinal delivery of lentiviral EIAV vector encoding endostatin and angiostatin for neovascular AMD has shown a well-tolerated safety profile with very minimal ocular inflammation.2 Moreover, intravitreal administration of an AAV2 viral vector encoding the gene for aflibercept, an anti-VEGF therapy, has exhibited highly encouraging outcomes in managing the disease activity of neovascular AMD[4],[8].
Glaucoma
In recent expansion of knowledge of disease pathology and gene variants, underlying genetic factors have been identified contributing to glaucoma[4]. This has allowed for the possibility of the use of viral gene vectors as therapeutic approaches. There are preclinical and phase I trials that have studied the use of siRNA in suppressing beta ß2-adrenergic-receptor synthesis in lowering intraocular pressure[4].
Corneal Diseases
Exploration of gene therapy as a potential treatment for the following both hereditary and non-hereditary corneal diseases is being explored in animal trials[4].
- Non-inherited
- Herpes Simplex Keratitis
- Sjogren's Syndrome
- Corneal graft rejection
- Corneal revascularization
- Inherited
- Mucopolysaccharidosis (MPS)
- Meesmann epithelial corneal dystrophy
- Ectrodactyly-ectodermal dysplasia-clefting (EEC) syndrome
- Aniridia
- Fuchs endothelial corneal dystrophy
Optic Nerve
Leber Hereditary Optic Neuropathy
Leber Hereditary Optic Neuropathy is marked by retinal ganglion cell death resulting from ND1 (G3460A), ND4 (G11778A), and ND6 (T14484C) gene mutations. It is an inherited mitochondrial condition, and therefore viral gene therapy has been targeted toward the mitochondrial genome. Phase I clinical trials have thus far shown safety and effectiveness of gene therapy employing AAV2 viral vectors encoding ND4[4].
Modes of Delivery
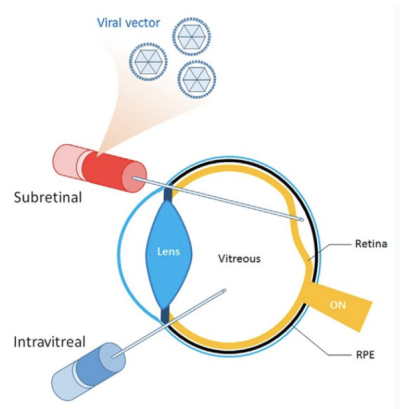
Injection of ocular viral gene therapy can be achieved through various methods, including intravitreal, subretinal, and suprachoroidal routes. Each delivery method carries its own set of both benefits and disadvantages, such as the impact on the immune system resulting in varying levels of inflammation.
Intravitreal
The intravitreal route is considered to be the safest and least invasive method[4][1][8]. However, the downside of this route is its limited capacity to transduce the outer retina, along with the strong humoral immune response that it causes. There have been reported cases of vision loss resulting from the excessive intraocular inflammation following administration of vectors via this route[4]. Interestingly, anterior segment inflammation is contingent upon the existence of a genome. In other words, viral particles with empty capsids, failed to elicit any reaction in the anterior segment. Nonetheless, the same viral particles with empty capsids, in addition to full viral particles, both cause vitreal inflammation after administered intravitreally. Although the viral particles with no genome did result in inflammation, it was to a lower extent than that of full viral particles. This indicates that capsids specifically caused inflammation in the vitreous, whereas genetic material resulted in inflammation in both the anterior and posterior segments of the eye[4].
The outer retina contains both the retinal pigment epithelium (RPE) and photoreceptor cells, both of which are the primary regions of where defects associated with retinal genetic diseases are located. The intravitreal route allows the vector to transduce the retina from the vitreous side, resulting in restricted capability to transduce the outer retina[4][1]. This consequently limits the use of intravitreal injections of viral vectors as a therapeutic method for a plethora of conditions. Moreover, this administration route produces a significant amount of antibodies, ultimately reducing the efficacy of treatment by attacking and eliminating transduced cells[4]. Intravitreal injection is further limited as a therapeutic method for conditions that require treatment of bilateral eyes. This is due to the observation that, as a result of neutralizing antibodies, injection in one eye ultimately inhibits the expression of viral vectors in the opposite eye, specifically when both eyes receive an intravitreal injection with the identical vectors[4].
Procedure
Intravitreal injections are typically performed in-office under local anesthesia using 2% lidocaine. Iodine is used to sterilize the ocular field. Approximately 3.5 mm posterior to the limbus, a 30 Gauge needle carrying the viral vector is positioned through the sclera at the pars plana [9].[2],[11]. The viral vector is then administered into the vitreous space.
Post-Op
Patients should avoid rubbing their eyes. Patients can use OTC artificial tears or lubricants to help with irritation and dryness. Subconjunctival hemorrhage is normal at site of injection and floaters are normal.
Adverse Events
Endophthalmitis and retinal detachment are the most common risks, but the complication rate is <1%. [2]
Subretinal
The subretinal route is the most invasive method of injecting viral vectors, requiring pars plana vitrectomy and then macular retinotomy via a small gauge cannula[4], [8]. The injection of the viral vector between the retinal pigment epithelium (RPE) and photoreceptor cells ultimately results in a bleb, causing separation of the two layers[1]. Although this method ensures transduction of the outer retina, there is risk of damage to retinal tissue. Perioperative care involves systemic steroids and the typical follow-up and course of action involved with vitrectomy and subretinal injection procedures[9]. Potential complications that may arise from this procedure include macular holes, subretinal hemorrhage, subretinal fibrosis, and retinal detachment[8]. Nonetheless, this route offers the most direct access to the outer retina, specifically the retinal pigment epithelium (RPE) and photoreceptor cells[6]. The benefit of subretinal delivery is the minimal to absent humoral response generated. The first and only currently approved ocular viral gene therapy, voretigene neparvovec (Luxturna), for treatment of Leber Congenital Amaurosis, is administered via the subretinal route[4],[8].
Procedure
Subretinal injections must be done as a surgery in an operating room under retrobulbar anesthesia or even under general anesthesia for those who are less pain tolerant. Iodine is used to sterilize the ocular field. A 23 Gauge or 25 Gauge needle is initially used to remove the vitreous and execute a pars plana vitrectomy[9]. A 41 Gauge tip is then positioned into the subretinal space in order to create a retinotomy and induce a bleb with a balanced salt solution infusion, followed by guided administration of the viral vector into the area[9][2]. An intraoperative OCT can help with localization and allow for maximum precision of vector delivery.
Post-Op
In order to contain the subretinal fluid within the posterior pole, patients are typically instructed to remain supine for 2-24 hours. Oral prednisone is taken for 21-61 days after surgery, with a taper after the first 2 weeks[12]. Topical antibiotics and topical corticosteroids may be added for ocular inflammation. A macular OCT should be done on either postoperative day 1 or 7 depending on if the eye is fluid or air filled, respectively. Sequential follow-up should include evaluation of visual acuity, visual fields, microperimetry, electroretinogram, OCT, fundus photos, and autofluorescence[12].
Adverse Events
Smaller needle gauges have lower complication rates associated with them. The complications are associated with the surgical procedure itself, not the viral vector. Clinical trials with subretinal injection of Luxturna showed a complication rate consisting of 18% elevated IOP, 8% ocular inflammation, 5% epiretinal membrane, and 3% choroidal hemorrhage post-op[9]. Other possible complications includes outer nuclear or central retinal layer thinning, retinal tears, and retinal detachment.
Suprachoroidal
Suprachoroidal injection of viral vectors occurs in the space between the choroid and sclera. This method is a less invasive option compared to subretinal delivery. However, research is still limited with this delivery method of viral vectors, and more studies need to be done in order to learn more about its benefits and complications. There is currently one reported animal study that has presented evidence indicating the potential for safe and effective delivery of AAV to the retina through suprachoroidal administration[10][8]. Although this method can delivery viral vectors to the outer retina, current reports on the effectiveness of viral vectors injected via suprachoroidal routes to transduce photoreceptors still shows inconsistency. Another challenge is the lack of sustainability in gene expressions through this method, which has shown a gradual decline observed over a 3 month period[4]. This phenomenon may be linked to the heightened flow of choroidal circulation. An advantage to this delivery method is the weaker immune response created as compared to intravitreal administration [8],[4].
Procedure
Suprachoroidal injections are typically done in-office under local anesthesia using 2% lidocaine. First, a hypodermic microneedle is guided through the sclera posterior to the limbus. There is a syringe attached to the microneedle, and the viral vector is administered once there is no more resistance felt on advancement. Another technique utilizes an incisional sclerotomy followed by the advancement of a 250A microcatheter. However, this technique is more invasive and requires a surgical setting [13].
Post-Op
There is a need for further clinical studies and investigation.
Adverse Events
Research with this technique for viral vector gene therapy is still very limited. The study of AAV5 vector delivery via suprachoroidal injection was studied by Peden et al, which showed no complications of retinal detachment, retinal tears, endophthalmitis, hemorrhage, or other adverse reactions. [10]
Challenges
While significant progress and notable advancements have characterized the field of ocular viral gene therapy, there are also marked challenges and complexities that necessitate careful consideration.
Inflammation
The introduction of foreign DNA sequences and viral capsid proteins by the vector can be identified by the host cell as unfamiliar antigens. Specifically within the retina, microglia act as macrophages, potentially triggering inflammation. This poses a challenge as it can trigger acquired immunity, potentially neutralizing the intended therapeutic intent[3]. Current management incorporates the use of oral, periocular, or systemic corticosteroids to reduce inflammation. Approximately 10% of patients undergoing viral gene therapy exhibit noticeable ocular inflammation that requires treatment to some degree with steroids, yet a larger proportion of patients show subclinical inflammation[3].
The extent of ocular inflammation correlates with the dosage administered, with a greater risk following injection via the intravitreal route[3]. Ultimately, the severity of inflammation following viral vector gene therapy depends on the viral vector dose and route of administration[6]. Overall, corticosteroid treatment seems to be beneficial, but more research needs to be done in regards to both prevention and management of this ocular inflammation.
Insertional oncogenesis
Insertional oncogenesis occurs when transduced genetic material is unintentionally inserted close to proto-oncogenes, leading to their activation[4]. This is a risk with ocular viral gene therapy. The insertion of a viral genome can result in gene transactivation, providing transduced cells with a proliferative advantage[14]. Lentivirus integrates its complementary DNA into the chromosome of target cells, and thus has increased risk of insertional mutagenesis. There are multiple methods being employed to study the mitigation of this potential risk, one being the generation self-inactivating vectors by suppressing the 3’ end terminal repeat[8]. Unlike lentivirus, adenovirus and adeno-associated virus (AAV) remain episomal, meaning it is uncommon for them to integrate into the host cell genome and cause insertional mutagenesis[8].
Safety
Administering a subretinal injection is a highly invasive procedure involving a pars plana vitrectomy. Moreover, this method induces a subretinal bleb which may consequently cause transient detachment of the retinal pigment epithelium (RPE) from the photoreceptor cell layer, exacerbating degeneration and further increasing risks of complication such as muscular holes, choroidal effusions, retinal tears, or retinal detachments[6]. Although intravitreal injections are less invasive and cause less inflammation, this route has decreased capacity to transduce the outer retina. Additionally, the true extent of long-term expression and safety of viral vectors remains uncertain.
Cost
Significant economic obstacles are associated with this novel ocular therapy. There are substantial production costs that arise from the still limited yield of vectors. For instance, Luxturna is marketed for $425,000 per eye in the United States[6].
Prognosis and Outcomes
Although more research needs to be done, there have been positive prognostic outcomes associated with ocular gene therapy trials. For instance, patients with Leber Congenital Amaurosis experienced lasting clinical improvement for a duration of three years following only one injection using AAV2 viral vector.
Assessing the lasting impact of gene therapy over years to decades at this stage poses considerable challenges since designs, vectors, and visual enhancements differ amongst early phase I-II trials. For example, while one study using AAV2 as a viral vector demonstrated reduction in therapeutic effect and persistent degeneration of visual function after therapy, a differing study revealed stable visual function 4 years posttreatment in the Phase 1 study and 5 years in the Phase III study[15]. There is limited long-term clinical evidence, but ongoing post-approval safety studies are gathering long-term data to enhance our understanding of the treatment response durability.
The approval of Luxturna for treatment of Leber Congenital Amaurosis marked a significant milestone in the field of viral gene therapy. Serotype 1 adeno-associated virus (AAV2) vector, encoding the RPE65 gene, is administered into subretinal space following a vitrectomy[14]. There were 37 pediatric patients who had the AAV2 viral vector administered in at least 1 eye, and in every patient functional mobility and light-sensitivity testing remain stable for 4 years[9]. The only complication consisted of one patient who developed endophthalmitis[9]. The treatment was demonstrated to be both safe and effective, resulting in noticeable clinical improvements that enhanced patients’ capacity to navigate in low light conditions.
Moreover, immunogenic response to the AAV2 capsid in the contralateral eye did not pose an issue, thus allowing for bilateral treatment[2]. In a continued trial, 93% of individuals undergoing Phase III Luxturna treatment demonstrated improved functional vision based on Multi-Luminance Mobility Test assessments during the one year follow-up period. Furthermore, 72% of Phase III trial participants completed the Multi-Luminance Mobility Test under the lowest evaluated light level after one year[16].
Future Research
Presently, research findings and clinical protocols offer conflicting perspectives on the optimal strategies for managing inflammation. The extent of cellular immune activation that could possibly hinder efficiency of viral gene therapy are still uncertain, and there is no supporting evidence to guide the selection of optimal immunosuppressive measures. The correlation between preceding or triggered immune responses to viral vectors and the consequences and outcomes remains elusive, and more thorough investigation still needs to be explored[3].
More research is currently being performed to study lentiviral vectors for transduction of large genes. There are currently two clinical trials, Phase I called StarGen and Phase II called UshStat, investigating the use of lentiviral vectors encoding ABCA4 and MYO7A genes respectively for treatment of Stargardt disease and Usher syndrome type IB[6]. While there has not been significant progress or improvement noted thus far, there is an ongoing effort to include younger patients in the earlier stages of disease[6]. Ongoing research is also exploring viral gene therapy beyond monogenic inherited diseases, extending to addressing retinal disorders such as wet age-related macular degeneration (AMD)[6].
References
Add text here
- ↑ Jump up to: 1.0 1.1 1.2 1.3 1.4 Shamshad A, Kang C, Jenny LA, Persad-Paisley EM, Tsang SH. Translatability barriers between preclinical and clinical trials of AAV gene therapy in inherited retinal diseases. Vision Res. 2023 Sep;210:108258. doi: 10.1016/j.visres.2023.108258. Epub 2023 May 25. PMID: 37244011; PMCID: PMC10526971.
- ↑ Jump up to: 2.0 2.1 2.2 2.3 2.4 Xu, David MD; Khan, M. Ali MD; Klufas, Michael A. MD; Ho, Allen C. MD. Administration of Ocular Gene Therapy. International Ophthalmology Clinics 61(3):p 131-149, Summer 2021. | DOI: 10.1097/IIO.0000000000000365
- ↑ Jump up to: 3.0 3.1 3.2 3.3 3.4 Chan YK, Dick AD, Hall SM, Langmann T, Scribner CL, Mansfield BC; Ocular Gene Therapy Inflammation Working Group. Inflammation in Viral Vector-Mediated Ocular Gene Therapy: A Review and Report From a Workshop Hosted by the Foundation Fighting Blindness, 9/2020. Transl Vis Sci Technol. 2021 Apr 1;10(4):3. doi: 10.1167/tvst.10.4.3. PMID: 34003982; PMCID: PMC8024774.
- ↑ Jump up to: 4.00 4.01 4.02 4.03 4.04 4.05 4.06 4.07 4.08 4.09 4.10 4.11 4.12 4.13 4.14 4.15 4.16 4.17 4.18 4.19 4.20 4.21 4.22 4.23 4.24 4.25 4.26 4.27 4.28 4.29 4.30 Ghoraba HH, Akhavanrezayat A, Karaca I, Yavari N, Lajevardi S, Hwang J, Regenold J, Matsumiya W, Pham B, Zaidi M, Mobasserian A, DongChau AT, Or C, Yasar C, Mishra K, Do D, Nguyen QD. Ocular Gene Therapy: A Literature Review with Special Focus on Immune and Inflammatory Responses. Clin Ophthalmol. 2022 Jun 3;16:1753-1771. doi: 10.2147/OPTH.S364200. PMID: 35685379; PMCID: PMC9173725.
- ↑ Jump up to: 5.0 5.1 Lebherz C, Maguire A, Tang W, Bennett J, Wilson JM. Novel AAV serotypes for improved ocular gene transfer. J Gene Med. 2008 Apr;10(4):375-82. doi: 10.1002/jgm.1126. PMID: 18278824; PMCID: PMC2842078.
- ↑ Jump up to: 6.0 6.1 6.2 6.3 6.4 6.5 6.6 6.7 6.8 6.9 Bordet T, Behar-Cohen F. Ocular gene therapies in clinical practice: viral vectors and nonviral alternatives. Drug Discov Today. 2019 Aug;24(8):1685-1693. doi: 10.1016/j.drudis.2019.05.038. Epub 2019 Jun 5. PMID: 31173914.
- ↑ Naso MF, Tomkowicz B, Perry WL 3rd, Strohl WR. Adeno-Associated Virus (AAV) as a Vector for Gene Therapy. BioDrugs. 2017 Aug;31(4):317-334. doi: 10.1007/s40259-017-0234-5. PMID: 28669112; PMCID: PMC5548848.
- ↑ Jump up to: 8.00 8.01 8.02 8.03 8.04 8.05 8.06 8.07 8.08 8.09 8.10 8.11 8.12 8.13 8.14 Rodrigues GA, Shalaev E, Karami TK, Cunningham J, Slater NKH, Rivers HM. Pharmaceutical Development of AAV-Based Gene Therapy Products for the Eye. Pharm Res. 2018 Dec 27;36(2):29. doi: 10.1007/s11095-018-2554-7. PMID: 30591984; PMCID: PMC6308217.
- ↑ Jump up to: 9.0 9.1 9.2 9.3 9.4 9.5 9.6 9.7 Mehta N, Robbins DA, Yiu G. Ocular Inflammation and Treatment Emergent Adverse Events in Retinal Gene Therapy. Int Ophthalmol Clin. 2021 Jul 1;61(3):151-177. doi: 10.1097/IIO.0000000000000366. PMID: 34196322; PMCID: PMC8259781.
- ↑ Jump up to: 10.0 10.1 10.2 10.3 10.4 Peden MC, Min J, Meyers C, Lukowski Z, Li Q, Boye SL, Levine M, Hauswirth WW, Ratnakaram R, Dawson W, Smith WC, Sherwood MB. Ab-externo AAV-mediated gene delivery to the suprachoroidal space using a 250 micron flexible microcatheter. PLoS One. 2011 Feb 11;6(2):e17140. doi: 10.1371/journal.pone.0017140. PMID: 21347253; PMCID: PMC3037961.
- ↑ Ochakovski GA, Bartz-Schmidt KU, Fischer MD. Retinal Gene Therapy: Surgical Vector Delivery in the Translation to Clinical Trials. Front Neurosci. 2017 Apr 3;11:174. doi: 10.3389/fnins.2017.00174. PMID: 28420956; PMCID: PMC5376580.
- ↑ Jump up to: 12.0 12.1 Davis, Janet L. MD, MA*; Gregori, Ninel Z. MD*; MacLaren, Robert E. MB, ChB, DPhil†; Lam, Byron L. MD*. Surgical Technique for Subretinal Gene Therapy in Humans with Inherited Retinal Degeneration. Retina 39():p S2-S8, October 2019. | DOI: 10.1097/IAE.0000000000002609
- ↑ Kansara V, Muya L, Wan CR, Ciulla TA. Suprachoroidal Delivery of Viral and Nonviral Gene Therapy for Retinal Diseases. J Ocul Pharmacol Ther. 2020 Jul/Aug;36(6):384-392. doi: 10.1089/jop.2019.0126. Epub 2020 Apr 7. PMID: 32255727; PMCID: PMC7404827.
- ↑ Jump up to: 14.0 14.1 Arsenijevic Y, Berger A, Udry F, Kostic C. Lentiviral Vectors for Ocular Gene Therapy. Pharmaceutics. 2022 Jul 31;14(8):1605. doi: 10.3390/pharmaceutics14081605. PMID: 36015231; PMCID: PMC9414879.
- ↑ Bart P. Leroy, M. Dominik Fischer, John G. Flannery, Robert E. MacLaren, Deniz Dalkara, Hendrik P.N. Scholl, Daniel C. Chung, Claudio Spera, Daniel Viriato, Judit Banhazi; Gene Therapy for Inherited Retinal Disease: Long-Term Durability of Effect. Ophthalmic Res 10 January 2023; 66 (1): 179–196. https://doi.org/10.1159/000526317
- ↑ Russell S, Bennett J, Wellman JA, Chung DC, Yu ZF, Tillman A, Wittes J, Pappas J, Elci O, McCague S, Cross D, Marshall KA, Walshire J, Kehoe TL, Reichert H, Davis M, Raffini L, George LA, Hudson FP, Dingfield L, Zhu X, Haller JA, Sohn EH, Mahajan VB, Pfeifer W, Weckmann M, Johnson C, Gewaily D, Drack A, Stone E, Wachtel K, Simonelli F, Leroy BP, Wright JF, High KA, Maguire AM. Efficacy and safety of voretigene neparvovec (AAV2-hRPE65v2) in patients with RPE65-mediated inherited retinal dystrophy: a randomised, controlled, open-label, phase 3 trial. Lancet. 2017 Aug 26;390(10097):849-860. doi: 10.1016/S0140-6736(17)31868-8. Epub 2017 Jul 14. Erratum in: Lancet. 2017 Aug 26;390(10097):848. PMID: 28712537; PMCID: PMC5726391.