Unilateral Coronal Synostosis
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
Introduction
Unilateral coronal synostosis (UCS) is a disorder resulting from the premature fusion of a coronal suture on one side of the skull. It occurs in approximately 1/10,000 live births.[1][2] UCS is most often non-syndromic, present in the absence of other premature sutural fusion, and accounts for 12%-24% of non-syndromic craniosynostosis. Alternatively, UCS may be present along with other sutural fusion in more complex disorders that include craniosynostosis, such as Muenke syndrome or cranio-fronto-nasal dysplasia.[3] There are six total cranial sutures that are unfused in a newborn; each suture separates membranous bones of the cranial vault and closes during the first two decades of life.[4] With UCS, the coronal suture prematurely fuses, resulting in compensatory head growth in the direction of unfused sutures, resulting in an abnormal head shape.[5][6] UCS impacts orbital growth, ocular alignment, and vision development in children. Common ophthalmic manifestations include strabismus, anisometropic astigmatism, and amblyopia.[7][2][8][9]
Genetics and other predisposing factors
Approximately 50% of cases of non-syndromic craniosynostosis result from sporadic genetic mutations.[10][11]Currently, the cause of non-syndromic UCS is unknown. However, a genetic association has been reported with FGFR3 and TWIST1.[5][12] The FGFRs initiate and regulate transcription of several genes in the prenatal stage.[13] TWIST is a transcription factor gene that directly affects BMP signaling of skull osteoblasts.[14] It is thought that the pre-activation of these transcription factors leads to premature fusion of cranial sutures.
In one study, 8-10% of cases of coronal synostosis reported a positive family history of craniosynostosis.[15] Environmental factors, such as exposure to teratogens, maternal smoking, intrauterine fetal head constraint, and oligohydramnios, may be predisposing factors.[4][5]
Clinical features
UCS is confirmed clinically by a set of physical characteristics and neuroimaging. However, children with deformational plagiocephaly (DP) can be misdiagnosed with UCS. DP (also known as occipital plagiocephaly) is a common asymmetrical flattening of the skull that constriction forces can cause during birth, muscular torticollis, or persistent pressure from supine positioning. DP is usually noticed during the first 4 -12 postnatal weeks.[16] The estimated incidence of DP is approximately 20-50% in 6-month-old children and is found more frequently in males.[16][17] It frequently resolves on its own, though sometimes it is treated with orthosis (helmeting). However, DP and UCS may be readily differentiated based on physical exam.
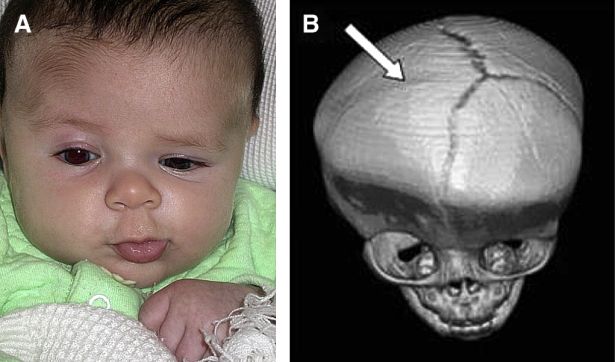
On clinical exam, UCS presents with “facial scoliosis” which includes a prominent ear on the affected side, ipsilateral flattening of the forehead, and nasal root deviation towards the affected side. The forehead on the side of the ipsilateral suture is retruded due to arrested growth, while compensatory growth occurs on the contralateral side of the forehead, which becomes more prominent (Figure 1). A common ophthalmic manifestation known as the “Harlequin deformity” (Figure 2) results from elevation of the ipsilateral superior orbital rim and lateral sphenoid wing, which results in eyebrow elevation and a larger palpebral fissure, as well as subtle proptosis.[9] This Harlequin deformity is also associated with a reduction in orbital volume; however, Kronig et al. found no association between the orbital volume ratio and the severity of UCS.[18]
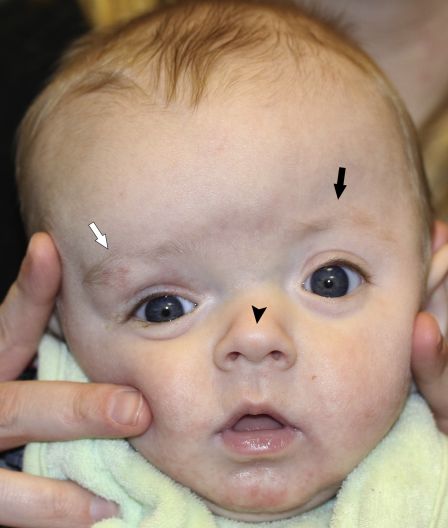
In contrast to patients with UCS, patients with DP have a parallelogram head shape, rarely demonstrate a "Harlequin" eye, and do not show a twist of the nasal root. DP can be managed with physical therapy and decreasing time spent leaning on the side of a flattened skull. If the condition is severe or if repositioning does not improve the appearance by six months of age, an orthotic helmet can be used to reshape the skull. In contrast, unlike DP, the skull deformation from UCS worsens over time due to compensatory cranial growth away from the fused suture. Treatment for UCS is typically surgical by either endoscopic strip craniectomy (ESC) performed during the first months of life or by frontal orbital advancement (FOA) closer to a year of age. If a dysmorphic cephalic shape is questioned, timely evaluation by those with expertise in craniosynostosis is essential as the surgical interventions for UCS differ, are highly dependent on the age at evaluation, and may impact ophthalmic outcomes in the long run.[9]
Ophthalmic manifestations
Refractive errors
Astigmatism in healthy children is fairly common.[19] Dobson et al. reported astigmatism of 1.00 diopter (D) or more in 25% of children aged 1-48 months.[20] Although astigmatism is common in typical children, there is a higher prevalence of aniso-astigmatism in children with UCS, with higher degrees of astigmatism in the eye contralateral to the fused suture.[8][21][22] Levy et al.[21] reported that 54% of UCS patients (21 out of 39) had at least 1 D of astigmatism, and more astigmatism was seen on the side opposite the fused suture (current reference 20. The concept of greater astigmatism on the side opposite the fused coronal suture is referred to as “aniso-astigmatism.” Luo et al. reported that 60% (18 out of 30) of UCS patients had ≥ 1D of astigmatism. Touze et al.[23] reported astigmatism to be significantly higher on the contralateral side with a refractive cylinder error of 0.97±1.06 versus 0.56±0.68 D on the ipsilateral side of the synostotic suture in a group of 28 UCS children (p= 0.03). Tarczy-Hornoch et al.[22] reported that 56% (25 out of 61 children) had amblyogenic anisometropia; of those, 79% had a greater refractive error contralateral to the coronal synostosis. The high prevalence of contralateral astigmatism is thought to result from downward displacement of the orbital roof on the side opposite the fused suture applying direct pressure to the globe and altering corneal curvature. Kronig et al.[24] utilized computerized tomography (CT) to measure orbital volume and found that the volume of the affected side was significantly lower (p=0.001). Lo et al.[25] established different height-to-width ratios in the eyes of UCS infants.
The choice of surgical intervention to treat UCS has been shown to impact refractive error. UCS patients treated by ESC versus FOA demonstrate no difference in mean aniso-astigmatism; however, the standard deviation in children treated by FOA was higher than in ESC.[26][27] In a large retrospective study of 120 UCS patients, both the incidence of significant and median aniso-astigmatism were higher in FOA-treated patients.[9]
Strabismus
The prevalence of strabismus in UCS patients ranges from 19-71% but is significantly higher compared to children without craniosynostosis, with a prevalence of 2-4%.[1][3][21][28] Common strabismus patterns found in patients with UCS include superior oblique (SO) palsy, inferior oblique (IO) overaction, hyper-elevation in adduction and under-depression in adduction resembling SO palsy, and IO overaction, and horizontal strabismus.[23][21][29][30][31] UCS has been highly associated with oblique muscle dysfunction, typically starting on the same side of synostosis and sometimes evolving into classic V-pattern strabismus involving both sides.[29] Macintosh et al.[29] reported IO overaction/SO underaction in 50.8% (30 of 59) of the UCS cases, and 76.5% had vertical strabismus in the primary position. Samara et al.[30] reported that 87% of their UCS patients with SO-palsy pattern manifest this strabismus ipsilateral to synostotic suture. Using magnetic resonance imaging (MRI), Touze et al.[32] confirmed excyclorotation of recti muscles in bony orbits of UCS patients and malpositioning of the SO trochlea. Levy et al.[21] reported that 49% of 39 UCS patients had greater ipsilateral fundus excyclotorsion consistent with either primary SO palsy or excyclorotation of the rectus muscles. Greater incidence of moderate to severe fundus excyclotorsion was found in FOA-treated patients versus ESC treated patients.[26] It has been hypothesized that SO palsy is characteristic of UCS patients due to the retro positioning of the superior orbital rim and shortening of the orbital roof resulting in a retro-displacement of the trochlea and underaction of the SO.[31][33] Trochlear displacement may also occur iatrogenically during FOA.[31]
Amblyopia
Due to the high prevalence of astigmatism and strabismus in children with UCS, amblyopia is common. Amblyopia prevalence has been reported in 35%-38% of the UCS population.[23][21] Several studies found an association between anisometropia and amblyopia in UCS patients.[22][23] Tarczy- Hornoch et al.[22] reported that 56% of patients (14 out of 25 children) with UCS had amblyogenic anisometropia, 79% on the side opposite the synostosis. Timely monitoring and intervention are necessary to prevent vision loss.
Eyelid and lacrimal system abnormalities
Eyelid anomalies are rarely reported in the UCS population, although parents often mistakenly believe that downward displacement of the contralateral brow is a form of ptosis. Epiblepharon was reported in 26%, and lagophthalmos in 7% of UCS children in one study.[34] Diamond et al. reported lateral canthal dystopia in 14% of 34 UCS patients, and nasolacrimal duct obstruction was reported in 12%.[35]
Anterior segment anomalies
Anterior segment anomalies such as microphthalmos, iris coloboma, corectopia, and anisocoria are not typical of isolated UCS but can be seen with syndromic, multisuture craniosynostosis.[36] In an examination of 34 UCS patients by Diamond et al., 1 case of choroidal coloboma and 1 case of microphthalmos were reported preoperatively.
Papilledema
The restriction in cranial growth from a synostotic suture may lead to elevated intracranial pressure (ICP).[8][37] Elevated ICP was recorded in 4%-42% of children with single suture craniosynostosis and most typically seen with sagittal synostosis.[16] Documented cases of papilledema in UCS patients are variable and relatively rare. In a study conducted by Van de Beeten, 0 cases of papilledema were found in 84 UCS patients.[38] Touze et al. reported 1 case of papilledema in 28 UCS patients.[23]
Optic atrophy
Chronically elevated ICP in patients with craniosynostosis may result in papilledema and optic atrophy. Optic atrophy is more common in syndromic craniosynostosis as multiple sutures are fused.[39] Dagi et al. found that 15% of 54 patients with various types of craniosynostosis had optic atrophy on fundus exam.[40] Optic neuropathy is uncommon with isolated UCS. Ntoula et al. reviewed 122 craniosynostosis children and found no optic atrophy cases among those with UCS patients (13% of their cohort).[3] In another study, one patient was reported to have optic nerve pallor out of 17 children with UCS.[41]
Management
The main objectives in treating UCS are to ensure normal brain development by allowing enough space for expansion within the skull. Moderate to severe cases of UCS can be treated surgically by early ESC followed by orthosis or later FOA. There is still debate, but growing evidence of better ophthalmic outcomes after earlier treatment by ESC. ESC is a minimally invasive technique, usually performed within the first 3-4 months of life, and is associated with reduced operative time, blood loss, and hospitalization time.[42] Endoscopic intervention is only recommended for children below six months of age as it is not usually effective thereafter. Therefore, a timely referral is imperative. FOA is a more invasive operation that includes an open craniotomy with reconstruction, usually recommended closer to a year of age or later. Treatment by FOA has been associated with more significant incidence and severity of strabismus, aniso-astigmatism, and amblyopia.
In a study of 37 UCS patients, 27% of children treated by FOA (26 patients) had severe strabismus, while none treated by ESC (11 patients) developed severe strabismus by two years of age.[26] A larger cohort of 43 UCS patients had similar outcomes, with 32% of FOA-treated children and none treated by ESC developed severe V-pattern strabismus at their final visit (p<0.0001).[27] Ten of the 22 treated by FOA developed amblyopia compared to 2 of 21 ESC-treated patients. A total of 9 of the 22 patients treated by FOA underwent strabismus surgery compared to only 2 of the 21 ESC-treated patients (p=0.03). The odds ratio of having strabismus surgery when comparing FOA to ESC with helmet therapy was 6.3:1. In the largest retrospective age-adjusted study of 120 UCS patients, half of whom had been treated with FOA and half with ESC, the prevalence of moderate-to-severe V-pattern strabismus was found to be substantially higher at about 18 months of age (31% in those treated by FOA versus 15% after treatment by ESC (p=0.07)) with a significant difference persisting between these two cohorts at five years of age (60% in FOA versus 35% in ESC p=0.02).[9] A total of 43% of FOA-treated patients required multiple strabismus surgeries compared to only 22% of those treated by ESC (p=0.02). The predicted odds ratio of requiring strabismus surgery was 2.8 in those treated by FOA versus ESC.
Several reports have demonstrated reduced rates of aniso-astigmatism in ESC-treated patients compared to those treated by FOA. In a retrospective case series evaluating 37 UCS-treated children, no statistically significant difference in mean aniso-astigmatism of FOA (mean follow-up of 22.5 months) and ESC (mean follow-up of 20.54 months) were reported. However, the magnitude of aniso-astigmatism measured was likely greater in FOA-treated patients (p< 0.01).[26] MacKinnon et al. examined 43 UCS patients treated by FOA (mean follow-up 21.5 months) and ESC (mean follow-up 23.5 months) and found that the mean aniso-astigmatism was equal in both groups. However, the standard deviation of aniso-astigmatism was higher in the FOA group (0.92) compared to the ESC group (0.51) (p=0.04).[27] Mackinnon et al. reported amblyopia in 10 patients treated by FOA (22 patients) versus two patients treated by ESC (21 patients) (p=0.002).[27] In a study of 120 UCS patients, Elhusseiny et al.[9] found that by 60 months of age, both the incidence of significant aniso-astigmatism (72% in the FOA group versus 46% ESC group p<0.0001) and median aniso-astigmatism (-0.75±1.1 in FOA group versus -0±0.5 in ESC group p< 0.001) were significantly higher in the FOA cohort. Higher rates of amblyopia in FOA-treated patients may result from greater severity of astigmatism, more strabismus, or both.
The improved outcomes found in ESC-treated patients compared to FOA-treated patients may be attributed to the difference in surgical approach or the timing of the operation. Endoscopically treatment is usually performed before four months of age, while FOA at greater than ten months of age. This suggests that earlier treatment may be critical as compensatory cranial and orbital changes may worsen during the first year of life prior to craniofacial intervention, and these increase the risk of associated astigmatism, amblyopia, and strabismus.
Conclusion
Ophthalmic UCS manifestations include increased rates of astigmatism, strabismus, and amblyopia. Therefore, timing, proper evaluation, and physician recognition are vital in the appropriate treatment of UCS.
References
- ↑ Jump up to: 1.0 1.1 Rostamzad P, Arslan ZF, Mathijssen IMJ, et al. Prevalence of Ocular Anomalies in Craniosynostosis: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine. 2022;11(4):1060.
- ↑ Jump up to: 2.0 2.1 Huynh EM, Elhusseiny AM, Dagi LR. Ophthalmic Manifestations of Unilateral Coronal Synostosis. Curr Eye Res. 2023 Jun 29:1-8.
- ↑ Jump up to: 3.0 3.1 3.2 Ntoula E, Nowinski D, Holmstrom G, Larsson E. Ophthalmological findings in children with non-syndromic craniosynostosis: preoperatively and postoperatively up to 12 months after surgery. BMJ Open Ophthalmol. 2021;6(1):e000677
- ↑ Jump up to: 4.0 4.1 Kajdic N, Spazzapan P, Velnar T. Craniosynostosis - Recognition, clinical characteristics, and treatment. Bosn J Basic Med Sci. 2018;18(2):110-116
- ↑ Jump up to: 5.0 5.1 5.2 Johnson D, Wilkie AOM. Craniosynostosis. European Journal of Human Genetics. 2011;19(4):369-376.
- ↑ Robinson S, Proctor M. Diagnosis and management of deformational plagiocephaly. J Neurosurg Pediatr. 2009;3(4):284-295.
- ↑ Rostamzad P, Arslan ZF, Mathijssen IMJ, et al. Prevalence of Ocular Anomalies in Craniosynostosis: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine. 2022;11(4):1060.
- ↑ Jump up to: 8.0 8.1 8.2 Elhusseiny AM, Dagi LR. (2022). Ophthalmic Complications of Craniosynostosis and the Impact of Endoscopic Repair. Endoscopic Craniosynostosis Surgery: An Illustrated Guide to Endoscopic Techniques, 71.
- ↑ Jump up to: 9.0 9.1 9.2 9.3 9.4 9.5 Elhusseiny AM, MacKinnon S, Zurakowski D, Huynh E, Dagi LR. Long-term ophthalmic outcomes in 120 children with unilateral coronal synostosis: a 20-year retrospective analysis. J AAPOS. 2021 Apr;25(2):76.e1-76.e5.
- ↑ Kajdic N, Spazzapan P, Velnar T. Craniosynostosis - Recognition, clinical characteristics, and treatment. Bosn J Basic Med Sci. 2018;18(2):110-116.
- ↑ Johnson D, Wilkie AOM. Craniosynostosis. European Journal of Human Genetics. 2011;19(4):369-376.
- ↑ Johnson D, Wall SA, Mann S, Wilkie AOM. A novel mutation, Ala315Ser, in FGFR2: a gene–environment interaction leading to craniosynostosis? European Journal of Human Genetics. 2000;8(8):571-577.
- ↑ Azoury SC, Reddy S, Shukla V, Deng CX. Fibroblast Growth Factor Receptor 2 (FGFR2) Mutation Related Syndromic Craniosynostosis. Int J Biol Sci. 2017;13(12):1479-1488.
- ↑ Behr B, Longaker MT, Quarto N. Craniosynostosis of coronal suture in twist1 mice occurs through endochondral ossification recapitulating the physiological closure of posterior frontal suture. Front Physiol. 2011;2:37.
- ↑ Ciurea AV, Toader C. Genetics of craniosynostosis: review of the literature. J Med Life. 2009;2(1):5-17.
- ↑ Jump up to: 16.0 16.1 16.2 Dias MS, Samson T, Rizk EB, et al. Identifying the Misshapen Head: Craniosynostosis and Related Disorders. Pediatrics. 2020;146(3).
- ↑ Robinson S, Proctor M. Diagnosis and management of deformational plagiocephaly. J Neurosurg Pediatr. 2009;3(4):284-295.
- ↑ Kronig SAJ, Kronig ODM, Zurek M, Van Adrichem LNA. Orbital volume, ophthalmic sequelae and severity in unilateral coronal synostosis. Child's Nervous System. 2021;37(5):1687-1694
- ↑ Dobson V, Miller JM, Harvey EM, Mohan KM. Amblyopia in astigmatic preschool children. Vision Res. 2003;43(9):1081-1090.
- ↑ Dobson V, Fulton AB, Sebris SL. Cycloplegic refractions of infants and young children: the axis of astigmatism. Invest Ophthalmol Vis Sci. 1984;25(1):83-87.
- ↑ Jump up to: 21.0 21.1 21.2 21.3 21.4 21.5 Levy RL, Rogers GF, Mulliken JB, Proctor MR, Dagi LR. Astigmatism in unilateral coronal synostosis: incidence and laterality. J aapos. 2007;11(4):367-372.
- ↑ Jump up to: 22.0 22.1 22.2 22.3 Tarczy-Hornoch K, Smith B, Urata M. Amblyogenic anisometropia in the contralateral eye in unicoronal craniosynostosis. J aapos. 2008;12(5):471-476.
- ↑ Jump up to: 23.0 23.1 23.2 23.3 23.4 Touzé R, Paternoster G, Arnaud E, et al. Ophthalmological findings in children with unicoronal craniosynostosis. Eur J Ophthalmol. 2022:11206721221077548
- ↑ Kronig SAJ, Kronig ODM, Zurek M, Van Adrichem LNA. Orbital volume, ophthalmic sequelae and severity in unilateral coronal synostosis. Child's Nervous System. 2021;37(5):1687-1694
- ↑ Lo LJ, Marsh JL, Kane AA, Vannier MW. Orbital dysmorphology in unilateral coronal synostosis. Cleft Palate Craniofac J. 1996;33(3):190-197.
- ↑ Jump up to: 26.0 26.1 26.2 26.3 MacKinnon S, Rogers GF, Gregas M, Proctor MR, Mulliken JB, Dagi LR. Treatment of unilateral coronal synostosis by endoscopic strip craniectomy or fronto-orbital advancement: Ophthalmologic findings. J aapos. 2009;13(2):155-160.
- ↑ Jump up to: 27.0 27.1 27.2 27.3 MacKinnon S, Proctor MR, Rogers GF, Meara JG, Whitecross S, Dagi LR. Improving ophthalmic outcomes in children with unilateral coronal synostosis by treatment with endoscopic strip craniectomy and helmet therapy rather than fronto-orbital advancement. J aapos. 2013;17(3):259-265.
- ↑ Friedman DS, Repka MX, Katz J, et al. Prevalence of amblyopia and strabismus in white and African American children aged 6 through 71 months the Baltimore Pediatric Eye Disease Study. Ophthalmology. 2009;116(11):2128-2134 e2121-2122.
- ↑ Jump up to: 29.0 29.1 29.2 Macintosh C, Wall S, Leach C. Strabismus in unicoronal synostosis: ipsilateral or contralateral? J Craniofac Surg. 2007;18(3):465-469.
- ↑ Jump up to: 30.0 30.1 Samra F, Paliga JT, Tahiri Y, et al. The prevalence of strabismus in unilateral coronal synostosis. Childs Nerv Syst. 2015;31(4):589-596.
- ↑ Jump up to: 31.0 31.1 31.2 Elhusseiny AM, Huynh EM, Dagi LR. Evaluation and Management of V pattern Strabismus in Craniosynostosis. J Binocul Vis Ocul Motil. 2020;70(1):40-45.
- ↑ Touzé R, Heuzé Y, Robert MP, et al. Extraocular muscle positions in anterior plagiocephaly: V-pattern strabismus explained using geometric mophometrics. Br J Ophthalmol. 2020;104(8):1156-1160
- ↑ Bagolini B, Campos EC, Chiesi C. Plagiocephaly causing superior oblique deficiency and ocular torticollis. A new clinical entity. Arch Ophthalmol. 1982;100(7):1093-1096.
- ↑ Chung SA, Yun IS, Moon JW, Lee JB. Ophthalmic findings in children with non-syndromic craniosynostosis treated by expansion cranioplasty. J Craniofac Surg. 2015;26(1):79-83.
- ↑ Diamond GR, Katowitz JA, Whitaker LA, Bersani TA, Bartlett SP, Welsh MG. Ocular and Adnexal Complications of Unilateral Orbital Advancement for Plagiocephaly. Archives of Ophthalmology. 1987;105(3):381-385
- ↑ Quercia NL, Teebi AS. Craniosynostosis, ectopia lentis, and congenital heart defects: further delineation of an autosomal dominant syndrome with incomplete penetrance. Am J Med Genet. 2002;107(1):38-42.
- ↑ Elhusseiny AM, Dagi LR. Craniosynostosis: A Child with Recurrent Papilledema. InFundamentals of Pediatric Neuro-Ophthalmology: A Practical, Case-Based Approach to Diagnosis and Management 2023 Jun 24 (pp. 235-241). Cham: Springer International Publishing.
- ↑ van de Beeten SDC, Cornelissen MJ, van Seeters RM, et al. Papilledema in unicoronal synostosis: a rare finding. J Neurosurg Pediatr. 2019;24(2):139-144. Published 2019 May 17. doi:10.3171/2019.3.PEDS18624
- ↑ Fearon JA, Barrientos S, Ditthakasem K, Herbert M. Optic Nerve Atrophy in Syndromic Craniosynostosis. Plast Reconstr Surg. 2022;150(2):381e-386e.
- ↑ Dagi LR, Tiedemann LM, Heidary G, Robson CD, Hall AM, Zurakowski D. Using spectral-domain optical coherence tomography to detect optic neuropathy in patients with craniosynostosis. J AAPOS. 2014;18(6):543-549.
- ↑ Chieffo DPR, Arcangeli V, Bianchi F, et al. Single-suture craniosynostosis: is there a correlation between preoperative ophthalmological, neuroradiological, and neurocognitive findings?. Childs Nerv Syst. 2020;36(7):1481-1488. doi:10.1007/s00381-020-04521-w
- ↑ Jimenez DF, Barone CM, Cartwright CC, Baker L. Early management of craniosynostosis using endoscopic-assisted strip craniectomies and cranial orthotic molding therapy. Pediatrics. 2002;110(1 Pt 1):97-104

