Ultrasound Cycloplasty in Glaucoma
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
Background
The ultrasound cyclo-plasty (UCP) is a non-incisional procedure based on high-intensity focused ultrasound (HIFU) aiming to reduce the intraocular pressure (IOP) in glaucoma patients. It reduces the aqueous humor production and increases the aqueous humor drainage through selective coagulative necrosis of the ciliary body, and stimulation of supra-choroidal and trans-scleral portions of the uveo-scleral outflow pathway with precise focusing on the target zone. The UCP has been reported to have a favorable safety profile compared with cyclo-destructive methods.
Device
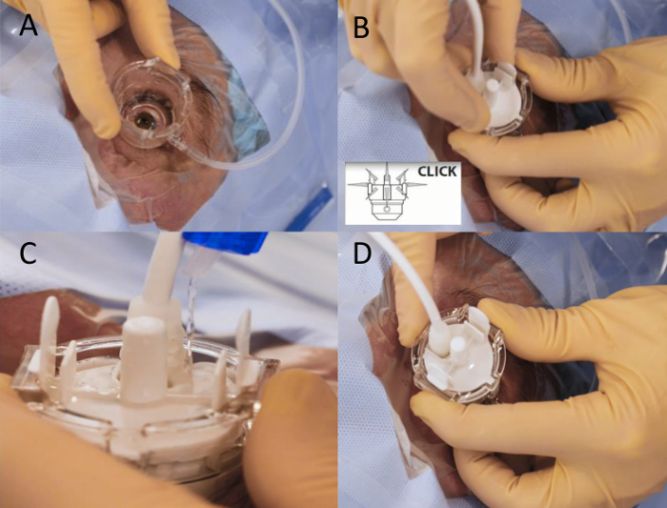
The UCP device has a ring probe that contains 6 piezoelectric transducers delivering ultrasound at 6 different places at different times. The procedure is usually performed in the operating room under peribulbar anesthesia. Figure 1 demonstrates the steps of the procedure. In brief, a coupling cone is first placed over the patient’s eye and maintained in its place by low suction to avoid its rotation during the procedure (Figure 1A). The ultrasound probe is then inserted in the coupling cone (Figure 1B-D). Throughout the whole procedure, the surgeon has to fill the space between the probe, coupling cone, and the patient’s eye with a balanced salt solution to allow proper delivery of the ultrasound waves. Each transducer is activated sequentially delivering ultrasound energy.[1][2]
Mechanism of action
The UCP reduces the aqueous humor production by thermal coagulation of the ciliary body inducing changes in the structure and vasculature of the ciliary processes without damaging adjacent tissues.[3] Additionally, the UCP increases uveoscleral aqueous humor outflow by increasing the intra-scleral outflow spaces.[4] However, a study by Nagar and colleagues demonstrated that the IOP lowering effects of UCP is mainly due to aqueous humor flow rate reduction rather than increased uveoscleral outflow.[5] Another study by Alaghband demonstrated a 15% reduction in the aqueous flow rate 3 months after HIFU, however, there was no significant change in the uveoscleral outflow.[6]
Outcomes
The UCP procedure was first described by Giannacare and colleagues. In their initial cohort, they included 10 open-angle glaucoma eyes with a mean age of 64.9 years at the time of treatment. They reported a significant reduction in the mean IOP from 24.8 ± 9.6 mmHg preoperatively on 3.9 ± 1.0 medications to a mean of 16.9 ± 2.8 mmHg on 1.9 ± 1.5 glaucoma medications at 1-year postoperatively. Visual acuity remained approximately stable throughout the 1-year follow-up. However, two patients did not have satisfactory IOP reduction and underwent an additional glaucoma surgery. Treatment tolerability was good, with no cases of hypotony or phthisis.[1]
Longfand retrospectively evaluated the safety and efficacy of UCP in 25 eyes with refractory glaucoma. They reported a significant reduction in the mean IOP from a baseline of 39.7 ± 6.1 mmHg on 2.4 ± 1.2 medications to 27.1 ± 11.0 mmHg on 2.3 ± 1.0 medications at 1 year. They demonstrated a lower success rate in the neovascular glaucoma patients compared to those with non-neovascular secondary glaucoma. The most common complications were conjunctival congestion, anterior chamber inflammation, and rarely scleral ring congestion.[7] Rouland and Aptel evaluated the long-term outcomes of UCP in 104 patients with refractory glaucoma. Surgical success was defined as a postoperative IOP of more than 5 mmHg and 20% IOP reduction from baseline without the development of any vision-threatening complications or the need for further glaucoma procedure. They reported significant reduction of the IOP from a mean of 27.6±8.9 mmHg preoperatively on 3 glaucoma medications to a mean of 15.6±4.3 mmHg on 2.8 glaucoma medications at 3-years with a success rate of 55%. They reported a low rate of complications with no cases of phthisis bulbi.[8]
In a prospective multicenter study including 66 eyes with primary open-angle glaucoma and no previous history of filtering surgery, Figus and colleagues reported a success rate, defined as IOP reduction of at least 20% without the development of vision-threatening complications or the need for glaucoma medications, of 74%. They did not report any major vision-threatening complication.[9]
Another prospective study by Giannaccare and colleagues evaluated the efficacy of UCP in 66 patients. Success was defined as a postoperative IOP <21 mmHg with (qualified) or without (complete) glaucoma medications. They reported a success rates of 68.1% (qualified) and 10% (complete) at 2-years postoperatively.[10]
Aptel et al. retrospectively studied 31 eyes who underwent a second session of UCP because of IOP rise before (early IOP rise group) or after (late IOP rise group) 6 months following the first UCP session. Success was achieved in 52.6% of the early IOP rise group and 55.5% in the late IOP rise group, suggesting that a second session of UCP can be effective in patients with unsatisfactory response to the first procedure.[11]
Yu and colleagues retrospectively compared the efficacy and safety of UCP and trans-scleral cyclophotocoagulation (TS-CPC) in patients with refractory glaucoma. They demonstrate no significant difference in the mean number of glaucoma medications, IOP reduction, or complications between both groups. However, postoperative pain at day 1 was significantly lower in the UCP group.[12] Another study by Ruixue and colleagues compared UCP to cyclocryotherapy in patients with neovascular glaucoma and demonstrated that in the cyclocryotherapy group, the ciliary body was completely destroyed while in the UCP group, coagulative necrosis affected only the ciliary epithelium.[13]
A large retrospective study including 182 eyes who underwent UCP with a mean follow-up of 29.71 months was conducted by Almobarak and colleagues. They reported a significant reduction in the mean IOP from 23.46 preoperatively to 16.24 mmHg postoperatively with a success rate of 85.6% at 24-months follow-up. Cataract development and AC reaction were the most common complications.[14]
A randomized controlled trial was conducted by Torky and colleagues to compare the outcomes of combined phacoemulsification and UCP to phacoemulsification alone in patients with visually significant cataract and co-existing open-angle glaucoma. The mean IOP reduction was significantly higher in the phaco-UCP group (median of 7 mmHg) compared to the phacoemulsification alone group (2 mmHg) at 18-months postoperatively. Qualified success, defined as postoperative IOP between 6 and 21 mmHg and more than 20% IOP reduction from baseline without additional glaucoma medications or surgery, was higher in the combined phaco-UCP group (67.7%) compared to phacoemulsification alone (16.7%) at last follow-up.[15] They reported no serious intra- or postoperative complications in any of the patients.
A meta-analysis found that ultrasound cycloplasty is a safe and effective technique that may reduce medication burden for glaucomatous individuals.[16]
Conclusion
UCP is a novel, simple, non-invasive glaucoma procedure that offers a reasonable IOP reduction with low rates of complications. Further prospective studies with long-term follow-up are still needed.
References
- ↑ Jump up to: 1.0 1.1 1.2 Giannaccare G, Sebastiani S, Campos EC. Ultrasound Cyclo Plasty in Eyes with Glaucoma. J Vis Exp. 2018 Jan 26;(131):56192.
- ↑ Posarelli C, Covello G, Bendinelli A, Fogagnolo P, Nardi M, Figus M. High-intensity focused ultrasound procedure: The rise of a new noninvasive glaucoma procedure and its possible future applications. Surv Ophthalmol. 2019
- ↑ Charrel T, Aptel F, Birer A, et al., Development of a miniaturized HIFU device for glaucoma treatment with conformal coagulation of the ciliary bodies, Ultrasound Med Biol, 2011;37:742–54.
- ↑ Mastropasqua R, Fasanella V, Mastropasqua A, et al., Highintensity focused ultrasound circular cyclocoagulation in glaucoma: a step forward for cyclodestruction?, J Ophthalmol, 2017;2017:7136275.
- ↑ Nagar A, Daas A, Danieliute L, Alaghband P, Yu-Wai-Man C, Amon A, Galvis E, Lim KS. Effect of high-intensity focused ultrasound (HiFU) treatment on intraocular pressure and aqueous humour dynamics: 12 -months results. Eye (Lond). 2021 Sep;35(9):2499-2505.
- ↑ Alaghband P, Galvis E, Ramirez A, Madekurozwa M, Chu B, Overby D, Lim KS. The Effect of High-Intensity Focused Ultrasound on Aqueous Humor Dynamics in Patients with Glaucoma. Ophthalmol Glaucoma. 2020 Mar-Apr;3(2):122-129.
- ↑ Longfang Z, Die H, Jie L, Yameng L, Mingyuan L, Xiaojing P. Efficacy and safety of single Ultrasound Cyclo-Plasty to treat refractory glaucoma: Results at 1 year. Eur J Ophthalmol. 2022 Jan;32(1):268-274.
- ↑ Rouland JF, Aptel F. Efficacy and Safety of Ultrasound Cycloplasty for Refractory Glaucoma: A 3-Year Study. J Glaucoma. 2021 May 1;30(5):428-435.
- ↑ Figus M, Posarelli C, Nardi M, Stalmans I, Vandewalle E, Melamed S, Skaat A, Leshno A, Sousa DC, Pinto LA. Ultrasound Cyclo Plasty for Treatment of Surgery-Naïve Open-Angle Glaucoma Patients: A Prospective, Multicenter, 2-Year Follow-Up Trial. J Clin Med. 2021 Oct 27;10(21):4982. doi: 10.3390/jcm10214982.
- ↑ Giannaccare G, Pellegrini M, Bernabei F, Urbini L, Bergamini F, Ferro Desideri L, Bagnis A, Biagini F, Cassottana P, Del Noce C, Carnevali A, Scorcia V, Traverso CE, Vagge A. A 2-year prospective multicenter study of ultrasound cyclo plasty for glaucoma. Sci Rep. 2021 Jun 16;11(1):12647.
- ↑ Aptel F, Tadjine M, Rouland JF. Efficacy and Safety of Repeated Ultrasound Cycloplasty Procedures in Patients With Early or Delayed Failure After a First Procedure. J Glaucoma. 2020 Jan;29(1):24-30.
- ↑ Yu Q, Liang Y, Ji F, Yuan Z. Comparison of ultrasound cycloplasty and transscleral cyclophotocoagulation for refractory glaucoma in Chinese population. BMC Ophthalmol. 2020 Sep 29;20(1):387. doi: 10.1186/s12886-020-01655-y.
- ↑ Ruixue W, Tao W, Ning L. A Comparative Study between Ultrasound Cycloplasty and Cyclocryotherapy for the Treatment of Neovascular Glaucoma. J Ophthalmol. 2020 Jan 22;2020:4016536.
- ↑ Almobarak FA, Alrubean A, Alsarhani WK, Aljenaidel A, Osman E. Ultrasound Cyclo Plasty in Glaucoma: Two-Year Outcomes. J Glaucoma. 2022 Jul 18.
- ↑ Torky MA, Alzafiri YA, Abdelhameed AG, Awad EA. Phaco-UCP; combined phacoemulsification and ultrasound ciliary plasty versus phacoemulsification alone for management of coexisting cataract and open angle glaucoma: a randomized clinical trial. BMC Ophthalmol. 2021 Jan 21;21(1):53.
- ↑ Wu TH, Yin X, Li JQ, Lu PR. Efficacy and safety of ultrasound cycloplasty for the treatment of glaucoma: a Meta-analysis. Int J Ophthalmol. 2023 Aug 18;16(8):1317-1325. doi: 10.18240/ijo.2023.08.19. PMID: 37602344; PMCID: PMC10398525.

