The Reliability of Intraocular Pressure Measurements
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
Glaucoma is disease defined by characteristic optic nerve findings and associated visual field dysfunction. While elevated intraocular pressure (IOP) is not pathognomonic for the disease, it remains critical in disease detection and management. To date, IOP is the only directly modifiable risk factor for glaucoma and the target for all therapeutic treatments, whether medication, laser, or surgery. This article aims to discuss the reliability of various techniques available to measure IOP.
Physiologic and Pathologic variation in IOP
Physiological and pathological variations in IOP have been extensively studied in the past few decades. For example, studies have shown that static measurements of IOP during regular clinic hours may not be sufficient to accurately depict the degree of fluctuation of IOP, as two-thirds of glaucoma patients exhibit their highest IOP values outside regular clinic hours, particularly during the nocturnal/sleep period and may vary considerably, by 10 mmHg or more, over a 24-hour period (1). Furthermore, seasonal variations in IOP have also been observed, with distinct seasonal variation of IOP in patients with normal-tension glaucoma over a 20-year study period (2). In another study, the difference in mean winter IOP was significantly higher than summer (3).
Other factors can also cause physiological and pathological variations in IOP. For instance, physical activity, body position, and changes in atmospheric pressure can all affect IOP readings. One study reported differences in IOP measurement when sitting, supine, and standing (4). Physical activity can also increase IOP which could lead to reduced ocular perfusion pressure (5).
Certain medical conditions can also cause variations in IOP. For example, patients with diabetes with elevated HbA1c levels have higher IOP readings compared to healthy individuals (6). Long term corticosteroid use has also been associated with increased IOP, with 2.8% of steroid users converting glaucoma (7). Additionally the use of antidepressants and stimulants also affect IOP. A single dose of fluoxetine, a popular antidepressant, was shown to increase IOP measurement (8). Both current and past smoking history also leads to an increase in IOP (9).
Palpation
Palpation is the earliest method for measuring IOP, only surpassed by instrumentation in the 20th century. It remains a simple and quick method for estimating IOP by directly pressing against the globe. Palpation is still used in situations where mechanical measurements are not possible, such as in patients who have had an artificial cornea. For example, the use of a pneumotonometer or handheld tonometer on the sclera in patients with a keratoprosthesis has been shown to overestimate IOP, while digital palpation may be more accurate (10). However, the reliability of this method is questionable because digital palpation has been demonstrated to only make crude assessments and distinguish whether the IOP is less or greater than 30mmHg. There is also little correlation between digital palpation and Goldmann tonometry readings (11). Furthermore, a study that compared palpation and Tono-Pen measurements after cataract surgery showed a mean difference between IOP readings of 10 mmHg. (12).
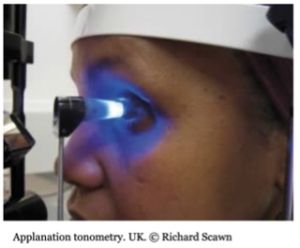
Applanation
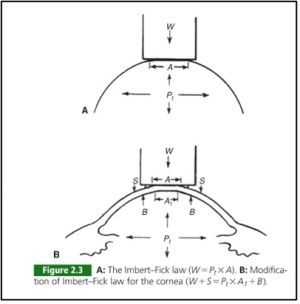
Applanation tonometry is the most prolific method for measuring IOP. Its direct contact with the cornea and determines IOP by utilizing the Imbert-Fick principle to measure the force applied by the device when a predetermined area of the cornea is flattened. (13)
The Goldmann and Perkins tonometers are the most widely used applanators and are considered the gold standard for IOP measurement. The Goldmann tonometer was introduced in 1954 and measures the force necessary to flatten a corneal area of 3.06mm diameter. The IOP equals the flattening force multiplied by 10. The Perkins tonometer is handheld and can be used without a slit lamp, making it more portable. However, the reliability of any applanation tonometer can be affected by various factors such as central corneal thickness (CCT) and corneal curvature.
Studies have shown that there is significant physician and technician variability in measurements, with two expert glaucoma physicians obtaining consecutive IOP measurements that differed greater than 2mmHg in 17% of eyes and even greater disagreement with consecutive technician measurements at 25% (14). Intraobserver and interobserver reliability in measurements have been reported to be 1.5 +/- 1.96 mmHg and 1.79 +/- 2.41 mmHg, respectively (14). In other words, the accuracy of any individual IOP measurement may vary by 3 or more mmHg simply based on the reliability limitations of the instrument.
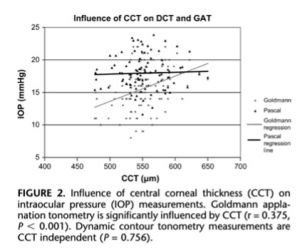
Several corneal parameters have importance when measuring IOP. Central Corneal Thickness (CCT) is also a significant factor in the accuracy of applanation tonometry, as the instrument was designed for an average corneal thickness of 520 microns. Patients classified as glaucoma suspects have been reported to have a higher CCT than individuals with open-angle glaucoma or healthy individuals, with 42% of glaucoma suspects having a CCT of greater than 585 microns. It has been estimated that 30-57% of elevated IOPs in glaucoma suspects are actually artifacts of measurement, and there is no universally accepted formula to "correct" IOP measurements for any given CCT. Based on a review of various correction-factor approaches, the range probably falls between 2.5 and 3.5 mmHg per 50 microns of difference from normal. The Ocular Hypertension Treatment Study (OHTS) showed that for those with a mean baseline IOP greater than 25.75 mmHg, the risk for glaucomatous damage at 5 years was 36% if the patient had a thin or average (555 micron) cornea and 13% with a CCT of 565 to 588 (15). A recent publication by Khawaja et al suggests that the association between CCT and glaucoma may be due to collider bias rather than a biological association. The findings suggest that CCT alone should not be used as a factor to identify people at high risk of glaucoma and using CCT in combination with IOP may be more effective (16).
There is evidence to suggest that corneal curvature may influence the accuracy of Goldmann applanation tonometry (GAT) in certain patient populations. For example, a study found GAT measurements were significantly influenced by corneal astigmatism (17). Similarly, another study reported GAT measurements were less reliable in eyes with moderate to high levels of astigmatism and recommended performing two separate measurements at 90 degrees apart to obtain a more accurate estimate of IOP (18). In contrast, some studies have suggested that corneal curvature may not have a significant influence on GAT or other tonometry methods, such as dynamic contour tonometry (DCT). For example, a study found corneal curvature affected IOP with DCT but not GAT measurements (19).
Corneal thickness also causes deviations in IOP with physiologically thick corneas overestimating and pathologically thick corneas underestimating the actual IOP (20). However, contact lens-induced corneal edema caused a small underestimation error in IOP measurements by the Pascal DCT and an overestimation error in Goldmann tonometry measurements (21). In post-surgical central corneal edema, no significant difference was found in IOP measurements using GAT and DCT before and 1 day after surgery (22).
Non-contact tonometry
The Ocular Response Analyzer (ORA) measures corneal hysteresis (CH) and IOP through a pneumatic mechanism with a column of air. After sufficient pressure is applied to applanate the cornea, the additional pressure needed to indent the cornea is used to calculate corneal elasticity and therefore CH. Algorithms are then used to adjust the measured IOP to compensate for the degree of measured CH. The ORA's reliability in measuring IOP was reported in a study to not be significantly associated with CCT, corneal curvature, or axial length (22). In another study comparing the reliability of ORA and GAT, the ORA was found to overestimate IOP in comparison to Goldmann applanation, with the degree of overestimation increasing at higher IOPs. (23).
Air-puff non-contact tonometry utilizes a column of air to applanate the cornea without need for topical anesthesia. It has been shown to overestimate IOPs in the lower range and underestimate IOPs in the higher range when compared to GAT.
Indention tonometry
Indentation tonometry involves creating a corneal deformation with a truncated cone. There are two types of indentation tonometry - Schiotz and Tonopen. The Schiotz indentation tonometer, introduced in 1905, was found to have better correlation to IOP obtained by Perkins applanation tonometer, especially when CCT was in the range of 501-550 μm (24). It is largely of historical reference, as it is rarely utilized in current practice.
The tonopen, however, is a very common instrument utilized in measuring IOP. Tonopen measurements were found to be affected by CCT, with reported errors of 0.29 mmHg per 10 microns in men and 0.12 mmHg per 10 microns in women. In addition, Tonopen readings may mask a third of eyes with elevated IOP and two-thirds of eyes with potentially above goal IOP, and it may not be interchangeable with GAT or sufficiently reliable for patient management or screening (25,26).
There are some studies that suggest that tonometry with Tonopen may be more reliable in patients who have had corneal surgery. One study found that Tonopen readings have greater reliability than GAT after LASIK surgery (27). Some studies have also suggested that Tonopen may underestimate IOP at low pressures and overestimate IOP at high pressures. One study found that Tonopen measurements were less reliable than Perkins tonometer in pressures greater than 16mmHg (28). Another study found that Tonopen measurements were lower in IOP less than 11mmHg and higher when IOP was greater than 11mmHg (29).
Rebound tonometry
A study on 141 eyes with glaucoma showed good correlation between rebound tonometry (RT) and GAT, although RT gave higher IOP measurements that increased as the IOP values correspondingly increased (30).
The iCare ic100 rebound tonometer showed excellent clinical reliability in terms of repeatability, interobserver reproducibility, and concordance after intravenous anesthesia in both control and LASIK groups. The reliability was independent of corneal parameters and axial length in both groups (31). However in another study, ICare and Tono-Pen XL significantly overestimated IOP compared to Perkins applanation tonometry. The mean difference between Perkins and ICare and Perkins and Tono-Pen XL was -3.35 +/- 2.28 mm Hg and -2.78 +/- 2.53 mm Hg, respectively (32). A study showed that iCare has correlation with GAT with 73% concordance within 5 mmHg (33). The iCare tonometer has also been studied as an effective and accurate method for measuring IOP at home, which allows for continuous IOP monitoring (33,34). A survey conducted by iCare home users showed that 73.7% of patients found it easy to use and 100% found it useful (34).
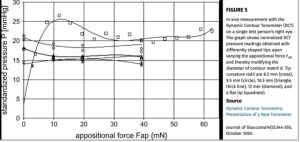
Pascal Dynamic Contour Tonometer
Although not widely used today, studies suggested this device can measure IOP with minimal impact from corneal characteristics (35,36).
Continuous IOP monitoring
New devices such as the SENSIMED Triggerfish® and Eyemate are designed to continuously measure the IOP. Studies have found weak correlation between the SENSIMED Triggerfish® CLS data output and IOP measurements taken using the Tono-pen® XL applanation tonometer. This suggests that the Triggerfish CLS may only provide data on relative changes in the IOP rather than absolute IOP. Additionally, the validity of Triggerfish CLS at estimating IOP compared to other methods is still controversial. (37, 38)
Eyemate is a micro-sensor embedded in a soft, biocompatible material in long-term medical implants to measure IOP. The device is found to be reliable, with a mean difference of -0.2 mmHg from GAT measurements and 100% of measurements within ±5 mmHg of GAT (39).
Interdevice variability of CCT
A study comparing CCT measurements using Scheimpflug, ultrasound, and optical coherence tomography found deviations within each device ranged from 5 to 15 microns, while differences between devices ranged up to 120 microns, with OCT measurements having the least deviation (40). Another study found a significant correlation between GAT and CCT, but not between DCT and CCT (41).
Conclusion
IOP measurements are crucial in the diagnosis and management of glaucoma, and there are significant limitations to the reliability of these measurements. While there are various methods for measuring IOP, conflicting evidence exists on the reliability of each. Physicians should be cognizant of this when utilizing their IOP measurements, and should consider compiling data when making clinical and surgical decisions.
References
1. Barkana Y,Anis S,Liebmann J,Tello C,Ritch R. Clinical utility of intraocular pressure monitoring outside of normal office hours in patients with glaucoma. Arch Ophthalmol. 2006;124(6):793
2. Ikeda Y, Mori K, Ueno M, Yoshii K, Nakano M, Sato R, Sato F, Maruyama Y, Imai K, Omi N, Yamamoto Y, Yamasaki T, Tashiro K, Sotozono C, Kinoshita S. Seasonal Variation and Trend of Intraocular Pressure Decrease Over a 20-Year Period in Normal-Tension Glaucoma Patients. Am J Ophthalmol. 2022 Feb;234:235-240. doi: 10.1016/j.ajo.2021.10.001. Epub 2021 Oct 12. PMID: 34648775.
3. Henmi T, Yamabayashi S, Furuta M, Hosoda M, Fujimori C, Tamura M, Kashiwagi F, Tsukahara S. [Seasonal variation in intraocular pressure]. Nippon Ganka Gakkai Zasshi. 1994 Aug;98(8):782-6. Japanese. PMID: 7942341.
4. De Bernardo M, Borrelli M, Cembalo G, Rosa N. Intraocular Pressure Measurements in Standing Position with a Rebound Tonometer. Medicina (Kaunas). 2019 Oct 18;55(10):701. doi: 10.3390/medicina55100701. PMID: 31635406; PMCID: PMC6843247.
5. McMonnies CW. Intraocular pressure and glaucoma: Is physical exercise beneficial or a risk? J Optom. 2016 Jul-Sep;9(3):139-47. doi: 10.1016/j.optom.2015.12.001. Epub 2016 Jan 12. PMID: 26794458; PMCID: PMC4911456.
6. Hymowitz MB, Chang D, Feinberg EB, Roy S. Increased Intraocular Pressure and Hyperglycemic Level in Diabetic Patients. PLoS One. 2016 Mar 22;11(3):e0151833. doi: 10.1371/journal.pone.0151833. PMID: 27002725; PMCID: PMC4803191.
7. François J. Corticosteroid glaucoma. Ann Ophthalmol. 1977;9(9):1075-1080.
8. Costagliola C, Parmeggiani F, Sebastiani A. SSRIs and intraocular pressure modifications: evidence, therapeutic implications and possible mechanisms. CNS Drugs. 2004;18(8):475-84. doi: 10.2165/00023210-200418080-00001. PMID: 15182218.
9. Lee CS, Owen JP, Yanagihara RT, Lorch A, Pershing S, Hyman L, Miller JW, Haller JA, Chiang MF, Lum F, Lee AY. Smoking Is Associated with Higher Intraocular Pressure Regardless of Glaucoma: A Retrospective Study of 12.5 Million Patients Using the Intelligent Research in Sight (IRIS®) Registry. Ophthalmol Glaucoma. 2020 Jul-Aug;3(4):253-261. doi: 10.1016/j.ogla.2020.03.008. Epub 2020 Mar 31. PMID: 33008558; PMCID: PMC7532983.
10. Netland, Peter A., Hisao Terada, and Claes H. Dohlman. "Glaucoma associated with keratoprosthesis." Ophthalmology 105.4 (1998): 751-757.
11. Baum J, Chaturvedi N, Netland PA, Dreyer EB. Assessment of intraocular pressure by palpation. Am J Ophthalmol 1995; 119:650 – 651.
12. Polk AJ, Nguyen V, Jarstad J. Is Palpation Sufficient for Estimation of IOP Immediately Following Cataract Surgery? Med Hypothesis Discov Innov Ophthalmol. 2020 Summer;9(2):143-148. Epub 2020 Mar 30. PMID: 32490021; PMCID: PMC7134245.
13. Jody Piltz-Seymour, M.D., Tak Yee Tania Tai, MD All authors and contributors: Sarwat Salim MD, FACS, Jody Piltz-Seymour, M.D., Ahmad A. Aref, MD, MBA, Oscar D. Albis-Donado, MD, Qi N. Cui, MD PhD, Vivian Qin. (2022, June 21). IOP and Tonometry. EyeWiki. Retrieved December 30, 2022, from https://eyewiki.org/IOP_and_Tonometry
14. Mihailovic A, Varadaraj V, Ramulu PY, Friedman DS. Evaluating Goldmann Applanation Tonometry Intraocular Pressure Measurement Agreement Between Ophthalmic Technicians and Physicians. Am J Ophthalmol. 2020 Nov;219:170-176. doi: 10.1016/j.ajo.2020.06.041. Epub 2020 Jul 5. PMID: 32640253.
15. Ocular Hypertension Treatment Study Group, European Glaucoma Prevention Study Group. Validated prediction model for the development of primary open-angle glaucoma in individuals with ocular hypertension. Ophthalmology 2007;114:10-19.
16. Khawaja AP, Jansonius NM. Potential for Collider Bias in Studies Examining the Association of Central Corneal Thickness With Glaucoma. Invest Ophthalmol Vis Sci. 2022 Nov 1;63(12):3. doi: 10.1167/iovs.63.12.3. PMID: 36322067; PMCID: PMC9639680.
17. Hagishima M, Kamiya K, Fujimura F, Morita T, Shoji N, Shimizu K. Effect of corneal astigmatism on intraocular pressure measurement using ocular response analyzer and Goldmann applanation tonometer. Graefes Arch Clin Exp Ophthalmol. 2010 Feb;248(2):257-62. doi: 10.1007/s00417-009-1202-7. Epub 2009 Sep 29. PMID: 19787365.
18. Papastergiou G.I., Kozobolis V., Siganos D.S. Effect of Recipient Corneal Pathology on Pascal Tonometer and Goldmann Tonometer Readings in Eyes after Penetrating Keratoplasty. Eur. J. Ophthalmol. 2010;20:29–34. doi: 10.1177/112067211002000104.
19. Francis BA, Hsieh A, Lai MY, Chopra V, Pena F, Azen S, Varma R; Los Angeles Latino Eye Study Group. Effects of corneal thickness, corneal curvature, and intraocular pressure level on Goldmann applanation tonometry and dynamic contour tonometry. Ophthalmology. 2007 Jan;114(1):20-6. doi: 10.1016/j.ophtha.2006.06.047. Epub 2006 Oct 27. PMID: 17070592.
20. Shah S, Chatterjee A, Mathai M, Kelly SP, Kwartz J, Henson D, McLeod D. Relationship between corneal thickness and measured intraocular pressure in a general ophthalmology clinic. Ophthalmology. 1999 Nov;106(11):2154-60. doi: 10.1016/S0161-6420(99)90498-0. PMID: 10571352.
21. Hamilton KE, Pye DC, Kao L, Pham N, Tran AQ. The effect of corneal edema on dynamic contour and goldmann tonometry. Optom Vis Sci 2008; 85: 451-456.
22. Medeiros FA, Weinreb RN. Evaluation of the influence of corneal biomechanical properties on intraocular pressure measurements using the ocular response analyzer. J Glaucoma 2006; 15:364–370.
23. Martinez-de-la-Casa JM, Garcia-Feijoo J, Fernandez-Vidal A, et al. Ocular response analyzer versus Goldmann applanation tonometry for intraocular pressure measurements. Invest Ophthalmol Vis Sci 2006; 47:4410–4414.
24. Nagarajan, S., Velayutham, V., & Ezhumalai, G. (2016). Comparative evaluation of applanation and indentation tonometers in a community ophthalmology setting in southern India. Saudi Journal of Ophthalmology, 30(2), 83–87. https://doi.org/10.1016/j.sjopt.2015.11.002
25. Bhan A, Browning AC, Shah S, Hamilton R, Dave D, Dua HS. Effect of corneal thickness on intraocular pressure measurements with the pneumotonometer, Goldmann applanation tonometer, and Tono-Pen. Investigative ophthalmology & visual science 2002; 43: 1389- 1392.
26. Blumberg, M. J., Varikuti, V. N., & Weiner, A. (2021). Real-world comparison between the Tonopen and Goldmann applanation tonometry in a university glaucoma clinic. International Ophthalmology, 41(5), 1815–1825. https://doi.org/10.1007/s10792-021-01742-z
27. Zadok D, Tran DB, Twa M, Carpenter M, Schanzlin DJ. Pneumotonometry versus Goldmann tonometry after laser in situ keratomileusis for myopia. J Cataract Refract Surg. 1999 Oct;25(10):1344-8. doi: 10.1016/s0886-3350(99)00202-3. PMID: 10511933.
28. Jaime Levy, Tova Lifshitz, Shirley Rosen, Zvi Tessler, Ben-Zion Biedner, Is the Tono-Pen Accurate for Measuring Intraocular Pressure in Young Children With Congenital Glaucoma, Journal of American Association for Pediatric Ophthalmology and Strabismus, Volume 9, Issue 4,2005, Pages 321-325,https://doi.org/10.1016/j.jaapos.2005.02.006.
29. Yasmin S. Bradfield, Brett M. Kaminski, Michael X. Repka, Michele Melia. Comparison of Tono-Pen and Goldmann applanation tonometers for measurement of intraocular pressure in healthy children. Journal of American Association for Pediatric Ophthalmology and Strabismus, Volume 16, Issue 3,2012,Pages 242-248, https://doi.org/10.1016/j.jaapos.2011.12.150.
30. Suman, Suwarna MS; Agrawal, Ajai MS; Pal, Virendra K. MS; Pratap, Vir B. MS. Rebound Tonometer: Ideal Tonometer for Measurement of Accurate Intraocular Pressure. Journal of Glaucoma 23(9):p 633-637, December 2014. | DOI: 10.1097/IJG.0b013e318285fefd
31. Gómez-Gómez A, Talens-Estarelles C, Alcocer-Yuste P, Nieto JC. Reliability of iCare ic100 Rebound Tonometry and Agreement With Goldmann Applanation Tonometry in Healthy and Post-myopic LASIK Patients. J Glaucoma. 2021 Aug 1;30(8):634-642. doi: 10.1097/IJG.0000000000001878. PMID: 33979108.
32. García-Resúa C, González-Meijome JM, Gilino J, Yebra-Pimentel E. Accuracy of the new ICare rebound tonometer vs. other portable tonometers in healthy eyes. Optom Vis Sci. 2006 Feb;83(2):102-7. doi: 10.1097/01.opx.0000200673.96758.7b. PMID: 16501412.
33. Turner, M., & Ou, Y. (2022). At-Home Glaucoma Monitoring: Is it Ready for Prime Time? Ophthalmology Glaucoma, 5(5), 580-582. https://doi.org/10.1016/j.ogla.2022.08.009
34. Hu, G. Y., Prasad, J., Chen, D. K., Alcantara-Castillo, J. C., Patel, V. N., & Al-Aswad, L. A. (2022). Home Monitoring of Glaucoma Using a Home Tonometer and a Novel Virtual Reality Visual Field Device: Acceptability and Feasibility. Ophthalmology Glaucoma, 5(4), 412-420. https://doi.org/10.1016/j.ogla.2022.05.001
35. Kanngiesser HE, Kniestedt C, Robert YC. Dynamic contour tonometry: presentation of a new tonometer. J Glaucoma 2005; 14: 344-350.
36. Özbilen KT, Kocabora MS. Measurement of Intraocular Pressure with Applanation, Dynamic Contour, and Air-Puff Tonometers: A Comparative Study in Primary Open-Angle Glaucoma and Healthy Cases. Beyoglu Eye J. 2020 Dec 28;5(3):178-187. doi: 10.14744/bej.2020.82713. PMID: 35098085; PMCID: PMC8784467.
37. Vitish-Sharma P, Acheson AG, Stead R, Sharp J, Abbas A, Hovan M, Maxwell-Armstrong C, Guo B, King AJ. Can the SENSIMED Triggerfish® lens data be used as an accurate measure of intraocular pressure? Acta Ophthalmol. 2018 Mar;96(2):e242-e246. doi: 10.1111/aos.13456. Epub 2017 Apr 9. PMID: 28391622.
38. Dunbar GE, Shen BY, Aref AA. The Sensimed Triggerfish contact lens sensor: efficacy, safety, and patient perspectives. Clin Ophthalmol. 2017 May 8;11:875-882. doi: 10.2147/OPTH.S109708. PMID: 28507427; PMCID: PMC5428792.
39. Szurman P, Gillmann K, Seuthe AM, Dick HB, Hoffmann EM, Mermoud A, Mackert MJ, Weinreb RN, Rao HL, Mansouri K; EYEMATE-SC Study Group. EYEMATE-SC Trial: Twelve-Month Safety, Performance, and Accuracy of a Suprachoroidal Sensor for Telemetric Measurement of Intraocular Pressure. Ophthalmology. 2022 Oct 3:S0161-6420(22)00760-6. doi: 10.1016/j.ophtha.2022.09.021. Epub ahead of print. PMID: 36202141.
40. Maloca PM, Studer HP, Ambrósio R Jr, Goldblum D, Rothenbuehler S, et al. (2018) Interdevice variability of central corneal thickness measurement. PLOS ONE 13(9): e0203884.
41. Schneider, E., & Grehn, F. (2006). Intraocular pressure measurement-comparison of dynamic contour tonometry and Goldmann applanation tonometry. Journal of Glaucoma, 15(1), 2–6. https://doi.org/10.1097/01.ijg.0000196655.85460.d6