Sustained Release Glaucoma Delivery Systems
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
Introduction
Topical glaucoma medications are pivotal in managing glaucoma, often necessitating concurrent use of multiple eye drops over extended periods.[1] Despite their efficacy in reducing intraocular pressure (IOP) and slowing glaucoma progression, they encounter several practical hurdles. Notably, poor adherence to therapy is prevalent, with up to 80% of patients failing to adhere to prescribed regimens.[2][3] Barriers to adherence include lack of social support, physical limitations hindering medication handling, forgetfulness, and inconvenient dosages, especially with multiple drops.[3] Furthermore, these medications, particularly those with preservatives, are associated with ocular surface issues, impacting quality of life.[4] Additionally, compared to glaucoma surgery, topical medications exhibit more significant IOP fluctuations, which may increase the risk of disease progression.[5] In addressing these challenges, sustained drug delivery systems have emerged as promising approaches, offering potential improvements in drug delivery and glaucoma management.
Drug Delivery Systems
A wide variety of sustained-release glaucoma drug delivery systems are currently developed or in the pipeline.
I. Punctal Plugs
A. Latanoprost Punctal Plug Delivery System (L-PPDS)
Design
L-PPDS (Evolute, Mati Therapeutics, Austin, TX, USA) is a punctal plug loaded with prostaglandin analogs. Designed to be placed intracanalicular in the same way as other commercial punctal plugs.
Efficacy and Safety
In a phase II clinical trial, Goldberg and Williams[6] enrolled 95 patients with open-angle glaucoma (OAG) or ocular hypertension (OHT) who had bilateral L-PPDS placement in both upper and lower puncta. At the 4-week follow-up visit, the mean baseline IOP was significantly reduced by 5.7 mmHg, and 87% did not report any discomfort.
B. OTX-TP intracanalicular insert
Design
Ocular Therapeutics (Bedford, MA, USA) designed a travoprost-loaded punctal plug to slowly release the medication over three months.
Efficacy and Safety
A Phase III multicenter trial compared OTX-TP insert to placebo in 554 OAG or OHT patients. There was no statistically significant difference in the mean IOP reduction between both groups at any time point. Hence, the device was discontinued.[7]
II. Conjunctival Fornix Inserts
A. Bimatoprost Ocular Insert
Design
The bimatoprost ocular insert (Allergan plc®, Dublin, Ireland) is a soft ring-shaped implant that is composed of 13 mg of preservative-free bimatoprost and silicone-matrix polymer placed over an inner polypropylene support to maintain its radial integrity. It is inserted into the upper and lower conjunctival fornices, either manually or using a scleral depressor, after the installation of topical anesthesia.[8]
Efficacy and Safety
In a phase II randomized controlled study, Brandt and colleagues[8] compared bimatoprost ocular insert to timolol 0.5% (administered twice daily) in 130 patients with either OAG or OHT for six months. They reported a significant reduction in the mean baseline IOP in the bimatoprost group of -3.2 to -6.4 mmHg compared to -4.2 to -6.2 mmHg in the timolol group, meeting the non-inferiority definition at 2 out of 9 time points. A follow-up open-label extension study[9] was then conducted and included two cycles of bimatoprost inserts over 13 months. They reported a waning effect of effectiveness over the first three months of follow-up, possibly due to prostaglandin receptor sensitization.
Both studies showed that bimatoprost implants are safe and well-tolerated. The retention rate was 88.5% at six months and maintained for 95% of individuals enrolled in the 13-month extension study.
B. Topical Ophthalmic Drug Delivery Device (TODDD)
Design
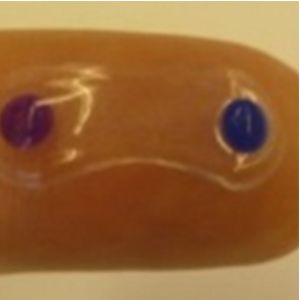
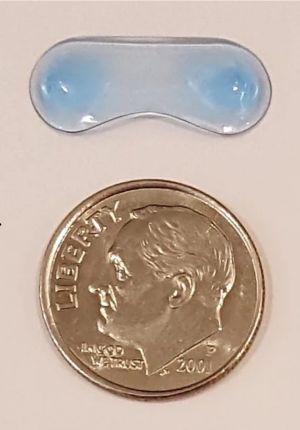
A soft polymer developed by Amorphex Therapeutics (Andover, MA, USA) is to be inserted in the superior conjunctival fornix. It provides continuous drug diffusion for over three months (Figures 1 and 2). It can be loaded with different drugs, including timolol, prostaglandin analogues, or a combination of both
Efficacy and Safety
Two animal studies have been conducted demonstrating its efficacy in IOP reduction. One study by Leahy and colleagues investigated the TODDD system loaded with timolol in normotensive rabbits over 14 weeks and showed significant IOP reduction (maximal IOP reduction was 5.5 mmHg, 37% IOP reduction from the baseline).[10] Another study investigated the use of the TODDD system loaded with 600µg in beagle dogs and showed 37% baseline IOP reduction. [11] In the only human study to date, including 14 patients, Leahy reported a retention rate of 70%.[12]
III. Contact Lenses
Design
Two drug-loaded contact lenses are currently under investigation. One is a latanoprost-loaded contact lens, and the other one is a preservative-free-bimatoprost-loaded contact lens (LL-BMT1, MediPrint Ophthalmics, San Diego, CA)
Efficacy and Safety
Ciolino and colleagues[13] have investigated the latanoprost-loaded contact lens. In an animal study on glaucomatous eyes of cynomolgus monkeys, there was a statistically significant greater IOP reduction with the contact lens-eluting latanoprost when compared to latanoprost ophthalmic solution on days 3, 5, and 8.
Phase II clinical trial compared LL-BMT1 to topical timolol (0.5%, twice daily). They reported a significant reduction of the mean baseline IOP of 14% from baseline at week one up to 19% from the baseline at week 3 with no serious adverse events.[14]
IV. Intracameral Implants
A. Intracameral Bimatoprost (Durysta)
Design
The bimatoprost implant (Durysta, Allergan, Irvine CA, USA) was approved by the Food and Drug Administration (FDA) on March 5th, 2020 for reduction of intraocular pressure (IOP) in patients with OAG or OHT. The implant is the first sustained-release therapy to be approved for this purpose. The bimatoprost implant is composed of biodegradable polymers designed to release bimatoprost in a non-pulsatile, steady-state manner over a 90-day period. In an animal study involving the treatment of dogs with the bimatoprost implant (15ug), 80.5% of the bimatoprost load was found to be released by day 51, and 99.8% had been released by day 80. Compared to topical dosing, concentrations of active drug were 4,400-fold higher at the iris-ciliary body with the bimatoprost implant.[15] Bimatoprost belongs to the prostaglandin analog (PGA) drug class and has been shown to lower IOP by increasing aqueous humor outflow via both the conventional trabecular route as well as the uveoscleral route.[16] Results from a study of normotensive beagle dogs that received the bimatoprost implant (30ug) suggest that the agent may also increase aqueous humor outflow by decreasing episcleral venous pressure.[17][18]
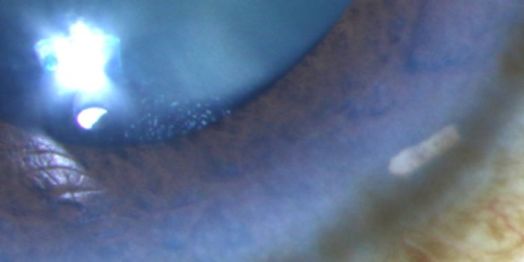
The bimatoprost implant is preloaded within a sterile applicator with a 28-gauge needle tip. Under aseptic conditions, the practitioner inserts the needle into the clear cornea, enters the anterior chamber, and then depresses an actuator button to release the implant (Video). Following the release of the implant, the needle is removed, and the patient is instructed to sit upright for at least one hour. The implant will then rest in the inferior anterior chamber angle (Figure 3).
Efficacy
The efficacy of the bimatoprost implant was investigated in two 20-month phase III trials involving 1,122 subjects that were randomized to treatment with the implant versus topical timolol eye drops.[19] Patients who were randomized to the bimatoprost implant had the drug administered in the study eye on day 1, week 16, and week 32. Patients randomized to topical timolol were treated with twice daily dosing. By week 12, patients randomized to the bimatoprost implant achieved an IOP reduction from 24.6 to 17.7 mmHg, representing approximately 30% reduction from baseline. The bimatoprost implant met pre-defined criteria for non-inferiority compared to timolol.
In a separate phase I/II dose-ranging study, 63 participants were randomized to 6, 10, 15, or 20ug bimatoprost implant administration in their study eye and topical bimatoprost once daily in their fellow (control) eye and followed for 24 months.[20] Eyes were permitted to receive a single repeat implant administration or rescue topical IOP-lowering medication during the study period. By 24 months, IOP was reduced from baseline by 7.5, 7.3, 7.3, and 8.9 mmHg in the 6, 10, 15, and 20ug bimatoprost implant groups, respectively. A single administration of the bimatoprost implant was found to control IOP in 40% of patients for up to 12 months and in 28% of patients for up to 24 months. In eyes treated with topical bimatoprost, the mean IOP reduction from baseline was 8.2mmHg at 24 months. The Durysta implant has shown efficacy in patients who have previously undergone glaucoma surgery.[21]
Retrospectively, Wong and colleagues reviewed the effects of Bimatoprost SR on IOP and the use of topical IOP-lowering medications in glaucoma patients, with a secondary focus on those with a history of selective laser trabeculoplasty (SLT).[22] They reported a significant reduction in mean IOP and the number of medications post-treatment compared to pretreatment levels, regardless of prior SLT history, suggesting the effectiveness of bimatoprost SR in managing glaucoma.[22] Recently, Choi and colleagues explored the impact of the bimatoprost implant on IOP and the requirement for topical IOP-lowering medications among glaucoma patients in clinical practice.[23] They analyzed data from 92 eyes treated at a tertiary eye center from November 2020 to October 2021, excluding cases where other IOP-lowering medications were added concurrently with the implant or had less than one month of follow-up. Although there was no significant change in IOP levels, the mean number of topical IOP-lowering medications notably decreased over the 12-month follow-up period, particularly for patients with mild to moderate glaucoma severity, despite a consistent total medication count, with additional interventions observed in a subset of cases during the follow-up period.
Safety
The bimatoprost implant is contraindicated in individuals with active or suspected ocular or periocular infections, corneal endothelial cell dystrophy, prior corneal transplantation, absent or ruptured posterior lens capsule, and in individuals with a history of hypersensitivity to bimatoprost.[24]
In the phase III studies evaluating the implant, the most common ocular adverse reaction was conjunctival hyperemia in 27% of patients. Other adverse reactions reported in 5-10% of patients included foreign body sensation, eye pain, photophobia, conjunctival hemorrhage, dry eye, eye irritation, increased intraocular pressure, corneal endothelial cell loss, blurred vision, iritis, and headache.[19]
B. Travoprost Implant (iDose TR)
Design
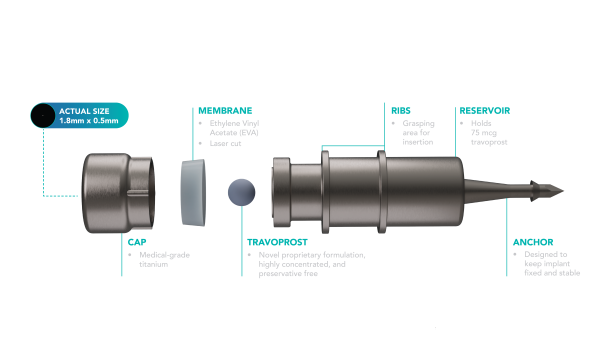
The iDose TR (Travoprost, Glaukos Corporation, Aliso Viejo, California, USA) comprises a titanium reservoir, approximately 0.5 millimeters wide and 1.2 millimeters long, enclosed within a single-dose inserter. Designed for sustained release, it delivers preservative-free travoprost (75 µg) through a nanoporous Ethylene Vinyl Acetate (EVA) membrane, with its thickness controlling the release rate (Figure 4). It comes in two types: fast-eluting (FE) and slow-eluting (SE), differing only in EVA membrane thickness, with the SE version available commercially and the FE model primarily for research. Surgical placement involves positioning it in the trabecular meshwork (Figure 5), secured with a 0.6-millimeter-long anchor attached to the sclera.
Efficacy and Safety
In their phase II trial, Berdahl and colleagues[25] investigated the safety and effectiveness of two implant designs compared to topical timolol 0.5% ophthalmic solution over three years. They found a significant decrease in mean baseline IOP, with reductions ranging from 7.3 to 8 mmHg in the SE design and 7.6 to 8.8 mmHg in the FE design versus 7.3 to 7.9 mmHg in the timolol group. Furthermore, a higher proportion of patients in the implant groups (63-69% at three years) achieved IOP control with the same or fewer medications compared to the timolol group (45% at three years), with a favorable safety profile observed in the implant groups. FDA approval was based on results from two multi-center randomized controlled trials (GC-010 and GC-012). In GC-010, Sarkisian and colleagues[26] enrolled 590 patients (197 in the SE- group, 200 in the FE group, and 193 in the topical timolol group). They demonstrated non-inferiority to timolol through 12 months, with significant reductions in mean IOP after SE-travoprost implantation ranging from 5.5 to 8.5 mmHg over a 12-month follow-up period.

Additionally, 81% of patients were medication-free at 12 months, and the implant showed high tolerability and a favorable safety profile, with most adverse ocular events associated with surgical implantation being mild and transient. Only one patient experienced endophthalmitis, which resolved with appropriate treatment. Notably, there were no instances of iris hyperpigmentation or periorbital fat atrophy, which are side effects associated with topical travoprost. Interestingly, conjunctival hyperemia was significantly lower in the implant groups compared to topical travoprost. Therefore, by delivering travoprost directly into the anterior chamber, intracameral implants effectively reduced or eliminated many side effects associated with topical prostaglandin analogs.[26][27]
C. OTX-TIC Intracameral Implant
OTX-TIC, developed by Ocular Therapeutix in Bedford, MA, USA, is integrated into a completely biodegradable implant administered into the anterior chamber using a 27 G or 26 G needle. This implant consists of travoprost-loaded microparticles embedded in the hydrogel, facilitating the prolonged release of travoprost throughout 4 to 6 months. Preclinical studies conducted on dogs revealed that target concentrations of travoprost were achieved in the aqueous humor, resulting in a sustained reduction in IOP and deemed safe.[28] Another study on rabbits monitored the release of travoprost from intracameral hydrogel implants over five months.[29] The findings indicated sustained high levels of travoprost in the aqueous humor for up to 4 months compared to travoprost eye drops. Furthermore, a randomized, double-blind, placebo-controlled Phase III clinical trial was specifically designed to assess the safety, durability, tolerability, and efficacy of OTX-TIC in glaucoma patients. Although there was a reduction in IOP ranging from 3.27 to 5.72 mm Hg across all time points from baseline, the trial did not meet its primary endpoint of achieving a statistically significant mean reduction in IOP compared to placebo at all nine time points.[30]
D. ENV515/Travoprost XR
Design
ENV515/Travoprost XR (Envisia Therapeutics, Morrisville, NC, U.S.A) is an implant injected into the anterior chamber, releasing travoprost over six months using a biodegradable system. Manufactured from poly(esteramide) (PEA) using PRINT® technology, the polymer is printed, filled with the active ingredient, and injected into the eye through a suitably sized needle. PEA undergoes intravitreal degradation, adhering to zero-order kinetics, thereby leading to the slow release of travaprost.[30]
Efficacy and Safety
In preclinical studies with normotensive beagle dogs, ENV515 insertion led to an average 35 ± 3% reduction in intraocular pressure (IOP) to 6.4 ± 0.6 mm Hg from baseline over 24 weeks, demonstrating a favorable safety profile and implant stability. In a Phase IIa clinical study involving patients with ocular hypertension, a single intracameral implantation of ENV515 resulted in a mean IOP reduction of around 6.7 ± 3.7 mm Hg (25% from baseline) after 11 months, with good tolerability but occasional dose-related transient hyperemia and eye redness.[31]
E. PA5108/Latanoprost FA SR
Design
The PA5108 (PolyActiva, Melbourne, Australia) is a rod-shaped biodegradable implant designed for insertion into the anterior chamber via a 27 G needle, ensuring a continuous 6-month release of latanoprost. Drug release begins immediately upon insertion, maintaining a consistent daily dose throughout the treatment period and achieving zero-order release profiles without any initial 'burst' effect. PolyActiva employs poly(ester), poly(triazole), or poly(urethane) systems that degrade independently or in combination to facilitate the release of the active drug. PA5108 comprises latanoprost acid covalently bonded to a monomeric polymer unit via a labile linker, with the polytriazole hydrogel polymer enabling medication release for 20 weeks before undergoing biodegradation.[32]
Efficacy and Safety
In a preclinical study involving ten glaucomatous dogs, various formulations of latanoprost implants, including PA5108, it demonstrated reduced IOP over 10, 19, and 34 weeks compared to latanoprost eye drops and placebo implants.[33]34 These implants were well-tolerated with no observed inflammation. A pending Phase I clinical trial aims to evaluate the safety and tolerance of PA5108, providing further insights into its efficacy and safety profile.[34]
V. Intraocular Lens Implants
Design
The SpyGlass system, developed by SpyGlass Pharma, is an innovative device designed for use during cataract extraction and intraocular lens (IOL) implantation procedures. It involves attaching drug-eluting pads to the optic-haptic junction of a hydrophobic single-piece intraocular lens. Subsequently, the intraocular lens, along with the drug-eluting pads, is inserted through a standard 2.4-mm clear corneal incision into the capsular bag. Importantly, the drug-eluting pads remain positioned outside the visual axis, slowly releasing medication for up to 3 years.[35]
Efficacy and Safety
Animal studies have shown significant IOP reduction, with a favorable safety profile. One study in humans including 23 indviduals with glaucoma or OHT reported promising results with mean IOP reduction of 45% from baseline at 9-months follow-up visit.[35] SpyGlass Pharma™ recently shared interim safety and efficacy data at the 12-month involving patients with glaucoma or ocular hypertension who received SpyGlass IOL-based Drug Delivery Platform with bimatoprost during cataract surgery.[36] Carried out at a single center in Latin America, the study comprised 23 subjects who completed the initial phase and transitioned into an extension study. Across three dosage cohorts (75 mcg, 150 mcg, and 300 mcg), participants were monitored over a planned three-year follow-up period, with safety and efficacy assessed through a battery of examinations. Findings revealed notable enhancements in visual acuity, alongside a substantial reduction in IOP. Specifically, mean IOP decreased from a baseline post-washout pressure of 25.1 ± 2.5 mmHg to 13.9 ± 2.3 mmHg (P<0.0001), with statistically significant reductions observed across all dosage cohorts at each assessment point. The average IOP reduction amounted to 44.6%, with 100% of eyes maintaining IOP levels ≤18 mmHg and experiencing a decrease in IOP exceeding 20% from baseline. Impressively, all eyes remained free from additional IOP-lowering medications throughout the study period. Adverse events were infrequent, predominantly mild in nature, and unrelated to the investigational product. This approach exhibited promising safety and efficacy profiles, underscored by substantial reductions in IOP and favorable visual acuity outcomes, while adverse events remained minimal and largely unrelated to treatment. Additionally, SpyGlass is actively enrolling patients in a Phase I/II study in the United States, aiming to further investigate the platform's performance in a larger patient cohort.
References
- ↑ Gedde SJ, Vinod K, Wright MM, et al. Primary Open-Angle Glaucoma Preferred Practice Pattern®. Ophthalmology. Jan 2021;128(1):P71-p150. doi:10.1016/j.ophtha.2020.10.022
- ↑ Olthoff CM, Schouten JS, van de Borne BW, Webers CA. Noncompliance with ocular hypotensive treatment in patients with glaucoma or ocular hypertension an evidence-based review. Ophthalmology. Jun 2005;112(6):953-61. doi:10.1016/j.ophtha.2004.12.035
- ↑ Jump up to: 3.0 3.1 Schwartz GF, Quigley HA. Adherence and persistence with glaucoma therapy. Surv Ophthalmol. Nov 2008;53 Suppl1:S57-68. doi:10.1016/j.survophthal.2008.08.002
- ↑ Scelfo C, ElSheikh RH, Shamim MM, Abbasian J, Ghaffarieh A, Elhusseiny AM. Ocular Surface Disease in Glaucoma Patients. Curr Eye Res. Mar 2023;48(3):219-230. doi:10.1080/02713683.2022.2041041
- ↑ Guo ZZ, Chang K, Wei X. Intraocular pressure fluctuation and the risk of glaucomatous damage deterioration: a Meta-analysis. Int J Ophthalmol. 2019;12(1):123-128. doi:10.18240/ijo.2019.01.19
- ↑ Goldberg DF, Williams R. A Phase 2 Study Evaluating Safety and Efficacy of the Latanoprost Punctal Plug Delivery System (L-PPDS) in Subjects With Ocular Hypertension (OH) or Open-Angle Glaucoma (OAG). Investigative Ophthalmology & Visual Science. 2012;53(14):5095-5095.
- ↑ Ocular Therapeutix announces tipline results of phase 3 clinical trial of OTX-TP for the treatment of glaucoma. News release. March 20th , 2024. https://www.biospace.com/article/releases/ocular-therapeutix-announces-topline-results-of-phase-3-clinical-trial-of-otx-tp-for-the-treatment-of-glaucoma/.
- ↑ Jump up to: 8.0 8.1 Brandt JD, Sall K, DuBiner H, et al. Six-Month Intraocular Pressure Reduction with a Topical Bimatoprost Ocular Insert: Results of a Phase II Randomized Controlled Study. Ophthalmology. 2016/08/01/ 2016;123(8):1685-1694. doi:https://doi.org/10.1016/j.ophtha.2016.04.026
- ↑ Brandt JD, DuBiner HB, Benza R, et al. Long-term Safety and Efficacy of a Sustained-Release Bimatoprost Ocular Ring. Ophthalmology. 2017/10/01/ 2017;124(10):1565-1566. doi:https://doi.org/10.1016/j.ophtha.2017.04.022
- ↑ Leahy CD, Ellis EJ, Ellis JY, Crawford KS. Efficacy of a Topical Ocular Drug Delivery Device (TODDD) for the Treatment of Glaucoma by Telemetric Measurement of IOP in the Normal Rabbit. Investigative Ophthalmology & Visual Science. 2007;48(13):5816-5816.
- ↑ Crawford K, Ellis J, Rulander J, et al. Sustained Delivery of Prostaglandin from Drug-Containing Depots Using Ocular Rings in Beagles. Investigative Ophthalmology & Visual Science. 2013;54(15):5073-5073.
- ↑ Leahy CD, Gutner R, Varney W, et al. Continuous Wear Non-Invasive Device for Sustained Ocular Drug Delivery. Investigative Ophthalmology & Visual Science. 2014;55(13):481-481.
- ↑ Ciolino JB, Ross AE, Tulsan R, et al. Latanoprost-Eluting Contact Lenses in Glaucomatous Monkeys. Ophthalmology. Oct 2016;123(10):2085-92. doi:10.1016/j.ophtha.2016.06.038
- ↑ MediPrint Ophthalmics announces promising results from its SIGHT-2 phase 2b group 1 clinical study. Accessed on April 8, 2024. https://mediprintlens.com/mediprint-ophthalmics-announces-promising-results-from-its-sight-2-phase-2b-group-1-clinical-study/.
- ↑ Seal JR, Robinson MR, Burke J, et al. Intracameral sustained-release bimatoprost implant delivers bimatoprost to target tissues with reduced drug exposure to off-target tissues. J Ocul Pharmacol Ther. 2019;35(1):50-57.
- ↑ Curran MP. Bimatoprost. A review of its use in open-angle glaucoma and ocular hypertension. Drugs Aging 2009;26(12):1049-1071.
- ↑ Lee SS, Burke J, Shen J, et al. Bimatoprost sustained-release intracameral implant reduces episcleral venous pressure in dogs. Veterinary Ophthalmology. 2018;21(4):376-381.
- ↑ Elhusseiny, Abdelrahman M., and Osamah J. Saeedi. "Episcleral Venous Pressure and Flow." Glaucoma Physician. Accessed on April 14th, 2024. https://glaucomaphysician.net/issues/2023/march/episcleral-venous-pressure-and-flow/
- ↑ Jump up to: 19.0 19.1 Safety and efficacy of bimatoprost sustained-release in patients with open-angle glaucoma or ocular hypertension. ClinicalTrials.gov Identifier NCT02250651.
- ↑ Craven ER, Walters T, Christie WC, et al. 24-month phase I/II clinical trial of bimatoprost sustained-release implant (bimatoprost SR) in glaucoma patients. Drugs. 2020;80:167-179.
- ↑ Bowers ME, Wong MK, Ventimiglia J, Nicknam RM, Moster MR, Pro MJ, Dale E, Kolomeyer NN, Lee D, Zheng CX. Effect of bimatoprost sustained-release intracameral implant on intraocular pressure and medication burden in patients with prior glaucoma surgery. J Fr Ophtalmol. 2023 Nov 3:S0181-5512(23)00477-1. doi: 10.1016/j.jfo.2023.07.016. Epub ahead of print. PMID: 37926661.
- ↑ Jump up to: 22.0 22.1 Wong MK, Bowers ME, Ventimiglia J, et al. Short-Term Outcomes of Bimatoprost Sustained-Release Intracameral Implant in Glaucoma. Journal of Glaucoma. 2023;32(9):738-743. doi:10.1097/ijg.0000000000002271
- ↑ Choi EY, Johnson NA, Stinnett S, Rosdahl J, Moya F, Herndon LW. The Effect of Bimatoprost Implant on Glaucoma Patients: An Observational Study. Journal of Glaucoma. 9900:10.1097/IJG.0000000000002368. doi:10.1097/ijg.0000000000002368
- ↑ DurystaTM [package insert]. Irvine, CA: Allergan USA, Inc.
- ↑ Berdahl JP, Sarkisian SR, Jr., Ang RE, et al. Efficacy and Safety of the Travoprost Intraocular Implant in Reducing Topical IOP-Lowering Medication Burden in Patients with Open-Angle Glaucoma or Ocular Hypertension. Drugs. Jan 2024;84(1):83-97. doi:10.1007/s40265-023-01973-7
- ↑ Jump up to: 26.0 26.1 Sarkisian SR, Ang RE, Lee AM, et al. Travoprost Intracameral Implant for Open-Angle Glaucoma or Ocular Hypertension: 12-Month Results of a Randomized, Double-Masked Trial. Ophthalmology and Therapy. 2024/04/01 2024;13(4):995-1014. doi:10.1007/s40123-024-00898-y
- ↑ Sarkisian SR Jr, Ang RE, Lee AM, Berdahl JP, Heersink SB, Burden JH, Doan LV, Stephens KG, Kothe AC, Usner DW, Katz LJ, Navratil T; GC-010 Travoprost Intraocular Implant Investigators. Phase 3 Randomized Clinical Trial of the Safety and Efficacy of Travoprost Intraocular Implant in Patients with Open-Angle Glaucoma or Ocular Hypertension. Ophthalmology. 2024 Feb 27:S0161-6420(24)00161-1. doi: 10.1016/j.ophtha.2024.02.022.
- ↑ Trevino L, Navratil T, Robeson R, et al. Intracameral conversion of travoprost to travoprost acid in the normotensive beagle dog model. Investigative Ophthalmology & Visual Science. 2014;55(13):5270-5270.
- ↑ Langh J, Blizzard CD, Driscoll A, et al. Effect of hydrogel persistence on pharmacodynamics and tolerability of OTX-TIC, travoprost intracameral implant in Beagles. Investigative Ophthalmology & Visual Science. 2020;61(7):1242-1242.
- ↑ Jump up to: 30.0 30.1 Al-Qaysi ZK, Beadham IG, Schwikkard SL, Bear JC, Al-Kinani AA, Alany RG. Sustained release ocular drug delivery systems for glaucoma therapy. Expert Opinion on Drug Delivery. 2023;20(7):905-919.
- ↑ Therapeutics E. Envisia Therapeutics’ Lead Product Candidate, ENV515 (travoprost XR), Achieves Primary Efficacy Endpoint in Phase 2A Glaucoma Clinical Trial. Press Release. 6 October 2015. 2017.
- ↑ Adams CM, Papillon JP. Recent developments for the treatment of glaucoma. Drug Delivery Challenges and Novel Therapeutic Approaches for Retinal Diseases. 2020:189-256.
- ↑ Komaromy AM, Koehl K, Harman CD, et al. Long-term intraocular Pressure (IOP) control by means of a novel biodegradable intracameral (IC) latanoprost free acid (LFA) implant. Investigative Ophthalmology & Visual Science. 2017;58(8):4591-4591.
- ↑ Safety and Tolerability of a Prostaglandin Ocular Implant for Treatment of Open Angle Glaucoma.Full Text View-ClinicalTrials.gov. Jul 27, 2018 [cited 2020 Apr 8]; Available from: https://clinicaltrials.gov/ct2/show/NCT03604328.
- ↑ Jump up to: 35.0 35.1 https://www.ophthalmologytimes.com/view/spyglass-pharma-kicks-off-phase-i-ii-clinical-trial-of-intraocular-drug-delivery-platform. Accessed on March 26th, 2024
- ↑ Katz GJ; Robles MA; Robles PJ, Kahook MY. Safety and Efficacy of a Long-Duration Sustained-Release Bimatoprost Implant in Eyes with Ocular Hypertension or Mild to Moderate Glaucoma. presented at: ASCRS; April 2024; BOSTON, MA, USA.