Superior Ophthalmic Vein Cannulation for Treatment of Cavernous Sinus-Dural Fistulas
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
Disease Entity
Cavernous sinus-dural carotid fistulas, also known as carotid-cavernous sinus fistulas (CCFs) are aberrant communications between the venous channels of the cavernous sinus and the meningeal branches of the internal carotid artery (ICA) and/or the external carotid artery (ECA).[1] Closure of the fistula is often required to resolve the symptoms associated with this disease presentation. Traditionally, closure is achieved through femoral transarterial or transvenous approaches. In patients where these approaches have failed, or for whom these approaches are deemed anatomically impossible, an alternative approach is necessary. Direct cannulation of the superior ophthalmic vein (SOV) through orbitotomy is an approach that has shown potent efficacy in resolving cavernous sinus-dural fistulas that are difficult to treat through other means.
Presentation of Cavernous Sinus-Dural Fistulas
Patients of any age or sex can develop dural CCFs, though most commonly these fistulas arise in middle-aged and elderly females. The factors that govern this discrepancy in the populations that develop dural CCFs are not well understood. However, certain factors that increase the risk of developing dural CCFs suggest some mechanisms that may underlie dural CCF pathogenesis. These include chronic hypertension, atherosclerotic disease, inherited collagenopathies (ex. Ehlers-Danlos syndrome), and pregnancy.
A common initial presenting symptom of a patient with dural CCF is a sudden paresis of an ocular motor nerve. However, the timeline and nature of symptom presentation varies along several variables including the size and location of the fistula, as well as the route and speed of blood flow. For example, posteriorly draining fistulas are typically asymptomatic. Those that become symptomatic often present with isolated cranial neuropathies, such as oculomotor, trigeminal, or facial nerve paresis. Due to the mass effect of the fistula on surrounding structures within the cavernous sinus, the cranial nerves that run within the cavernous sinus (i.e. CNIII, CNIV, CNV1, CNV2, and CNVI) are at greatest risk of damage. Another common symptom during presentation is ipsilateral orbital (sometimes described as ocular) pain. Severe symptoms of posteriorly draining dural CCFs include brainstem congestion and intracranial hemorrhage, though these sequelae are uncommon. Longstanding posteriorly draining dural CCFs can evolve to become anteriorly draining, which alters the presenting symptoms.
Anteriorly draining dural CCFs commonly manifest with orbital symptoms secondary to their involvement of the superior and inferior ophthalmic veins. The symptoms of an anteriorly draining dural CCF are typically less severe due to the slow blood flow rate that is common amongst these fistulas. Mild cases of anteriorly draining dural CCFs present with minor orbital congestion, often characterized by ipsilateral eye redness secondary to dilation of the conjunctival and episcleral veins. The tortuous corkscrew appearance of these dilated vessels is highly specific to CCFs. Additional symptoms include proptosis, swelling of the eyelids, and conjunctival chemosis. These, especially proptosis, may become severe enough as to cause diplopia. Anteriorly draining dural CCFs can also present as ocular motor nerve paresis, which also often manifests as diplopia. Further sequelae of orbital congestion, including ischemic optic neuropathy, secondary glaucoma, and chorioretinal dysfunction, lead to permanent vision loss in more than 20% of patients who develop CCFs.[2]
Diagnosis
The diagnosis of CCFs should be suspected based on the timing and collection of symptoms with which the patient presents but is confirmed by imaging. The precise location, size, and nature (direct vs. indirect) of a cavernous sinus fistula dictates the treatment choice, making thorough anatomical characterization essential for planning subsequent steps. Thorough examination of the orbit should be conducted in any patient with suspicion of CCF, including auscultation for orbital bruits, and tonometry to evaluate the symmetry of the ocular pulse amplitude. However, even when these tests are within normal limits, a patient with suspicion for CCF should undergo immediate imaging.[2] Multiple imaging methods can be used, including transorbital color Doppler ultrasonography (CDUS), CT scanning and CT angiography, MRI and MR angiography (MRA). MRI/MRA are superior to CT and CDUS. However, digital subtraction angiography (DSA) is the diagnostic gold standard for CCFs.[3] This procedure relies on X-ray images taken sequentially both before and after a contrast medium passes through the carotid arteries, thereby illuminating the location, size, and flow rate of fistulas.[4] Use of contrast medium may be contraindicated in specific patient populations, including those with kidney failure.[5] In these patients, alternative imaging techniques, including MRA, may be used. Alternative rationale for early use of MRI/MRA, CT/CTA, or CDUS includes ruling out alternatives in the differential diagnosis.
Relevant Anatomy of the Superior Ophthalmic Vein Relative to the Cavernous Sinus
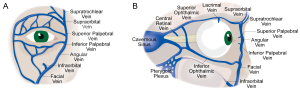
The SOV provides the primary veinous drainage from the superior orbit. This vein originates at the joining of the angular, supraorbital, and supratrochlear veins, just posterior to the trochlea (Figure 1A). The SOV then runs posterolaterally, inferior to the superior rectus muscle belly but superior to the optic and ciliary nerves. Along this route, several venous tributaries join the SOV, including the anterior ethmoidal, central retinal, lacrimal, medial ophthalmic, and superior vortex veins. After traversing the superior orbital fissure, the SOV drains into the cavernous sinus (Figure 1B).[6] [7]
Carotid-Cavernous Fistula Classification
Because the treatment of CCFs is heavily dependent on the characteristics of the fistula, namely the size, location, and direction and speed of blood flow, numerous classification methods have been devised to characterize CCFs. There is significant overlap between these classification systems. However, each system provides a slightly different perspective on the CCF. The most basic classification system for CCFs is based on the anatomical connections through which the fistula is created. Direct CCFs form from direct connections between the cavernous ICA and the cavernous sinus. These CCFs often present acutely and with severe symptoms. Indirect or dural CCFs are formed from connections between the cavernous sinus and branches of the ICA, or the ECA and its branches. These CCFs are less severe in onset and occasionally resolve without treatment. This anatomical classification can be further divided using the Barrow classification system.[8] This system divides CCFs into four anatomical types:
- Type A fistulas are those that form direct connections between the ICA and cavernous sinus.
- Type B fistulas are those that form connections between the dural branches of the ICA and the cavernous sinus.
- Type C fistulas are those that form connections between the dural branches of the ECA and the cavernous sinus.
- Type D fistulas are those that form connections between both dural ICA and dural ECA branches and the cavernous sinus.
Alternatively, CCFs can be classified by the anatomy of their venous drainage. The Thomas classification system[9] classifies CCFs using the following types:
- Type 1 fistulas drain exclusively posteriorly/inferiorly.
- Type 2 fistulas contain both posterior/inferior and anterior drainage.
- Type 3 fistulas drain exclusively anteriorly.
- Type 4 fistulas exhibit retrograde drainage into cortical veins and may contain other routes of venous drainage.
- Type 5 fistulas exhibit high-flow direct shunting between the cavernous ICA and the cavernous sinus and may contain other routes of venous drainage.
CCFs can be further classified by their etiology. Fistulas, especially direct CCFs can arise in response to head trauma, whereas indirect CCFs more commonly arise spontaneously as a result of long-standing vascular risk factors, including atherosclerosis, diabetes, and hypertension.[1]
Finally, CCFs can be classified by their hemodynamic flow rates. Using this metric, CCFs are typically designated as low or high flow. Commonly, direct CCFs are designated as high-flow fistulas, whereas indirect CCFs are designated as low-flow fistulas.[10]
Management
Non-Endovascular Management
Along with the anatomical and etiological considerations, the symptom burden of the patient is essential in determining management approaches. Asymptomatic, incidentally found CCFs are observed with a low threshold for intervention if symptoms arise. Especially when incidental CCFs are indirect in nature, they have been shown to self-resolve at high rates.[11] Symptomatic but non-emergent CCFs, may be treated with conservative topical approaches.[2][12] An additional consideration is the overall patency of the circle of Willis in the patient. Because dural CCFs typically arise in older patients with histories of atherosclerosis, diabetes and hypertension, procedures that increase the risk of further occluding collateral circulation may be life-threatening and are avoided if possible.[13]
Endovascular Management
In most cases, endovascular treatment is the preferred approach to obliterating symptomatic CCFs. Endovascular embolization techniques can be further divided into arterial and venous approaches.
Transarterial embolization involves entering the cavernous sinus through the ICA, and subsequently employing embolic methods, including coils, detachable balloons, liquid adhesives, and polyvinyl alcohol particles to obliterate the fistula. Especially when treating large fistulas, more than one approach may be necessary to successfully close the CCF.[14] [15] This approach is most effective in treating direct, high-flow CCFs. Severe adverse events have been reported as a consequence of this treatment, including migration of the embolic material to other intracranial vessels, as well as formation of arterial pseudoaneurysms.[16] [17] To avoid these risks, patients are concomitantly treated with anticoagulation and antiplatelet therapies.[14] Importantly, transarterial embolization is not well suited for treating dural CCFs, as the small meningeal arterial branches that are involved in the fistula are difficult to enter using this approach. Moreover, dural fistulas are often comprised of multiple arterial branches, further complicating a transarterial approach to embolization.[18]
Dural CCFs can more adequately be treated with transvenous embolization approaches. These commonly rely on access through transjugular or transfemoral routes, which pass through the inferior petrosal sinus prior to entering the cavernous sinus.[19] [20] Direct canulation of the SOV is also commonly used as an approach to the cavernous sinus.[21] This approach is described in greater detail in the following section. Similar to transarterial approaches, embolization is achieved through the use of coils, cyanoacrylate glue, absorbable gelatin sponges, detachable balloons, or polyvinyl alcohol particles. Patients undergoing transvenous embolization often report an initial worsening of symptoms prior to improvement.[22] Severe adverse events, including migration of embolic material, intracerebral hemorrhage, and sinus rupture have been reported.[2][20][23] [24] Importantly, combination therapies in which both transarterial and transvenous approaches are used may be necessary, especially in cases in which the CCF is large or when the CCF is comprised of numerous dural vessels.
In cases in which treatment is necessary, but endovascular approaches are not amenable, surgical obliteration of the CCF is required.[25]
Direct Cannulation of the Superior Ophthalmic Vein
Though the majority of patients presenting with CCFs can be treated with standard endovascular approaches, a subset of these patients either have anatomy that is incompatible with standard transarterial or transvenous approaches, or have previously been treated with standard approaches, which have failed to resolve the symptoms of the CCF. An alternative transvenous approach to the cavernous sinus can be used in these patients, which leverages the dilated nature of the SOV in patients with orbital congestion secondary to an anteriorly draining dural CCF. In 1989, Hanneken et al. demonstrated the efficacy of this approach in four patients who were not amenable to transarterial or other transvenous approaches. Though this approach had been attempted prior to their publication[26], they advanced the use of this technique by deciding to straighten the normally tortuous dissected SOV prior to catheterization, which allowed clearer access to the cavernous sinus and effective balloon embolization.[13] Subsequent to this publication, use of direct canulation of the SOV has grown considerably, and is now a viable approach to closure of atypical dural CCFs.[21][27] [28] [29] [30] [31] [32] [33] [34] [35] The procedure is performed in conjunction between the orbital surgeon and neuro-interventional radiologist. Prior to operating on a patient using direct canulation of the SOV, patients should be made fully aware of the risks and benefits of the approach. Though this approach is historically less common than transarterial approaches and transvenous approaches through the inferior petrosal sinus, direct canulation of the SOV has resulted in high success rates without morbidity.[13][21][27]
The initial dissection to the SOV is performed by the orbital surgeon:
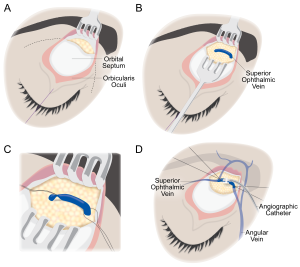
1. The skin is incised, a subbrow or lid crease incision may be used. Dissection is then carried along the suborbicular fascial plane, above orbital septum to the arcus marginalis (Figure 2A).
2. Using blunt dissection, the SOV is identified in the superomedial orbit lateral to the trochlea and isolated from the orbital fat (Figure 2B). The SOV is typically dilated and appears distinct from the surrounding fat.
3. The SOV is then stabilized prior to venipuncture through the use of two 2-0 silk ligatures (Figure 2C).
4. Once stabilized, an angiographic catheter (typically 18 gauge) is inserted directly into the SOV and then capped (Figure 2D). The SOV typically travels from medial to lateral, however anatomical variations exist, and the dilated SOV may form loops, which result in reversed venous configuration, and thus if the cannula does not pass easily from medial to lateral, then an attempt to pass it from lateral to medial should be made.
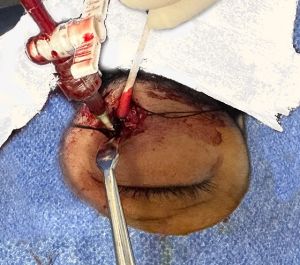
5. The catheter is then secured in place by tying 4-0 silk sutures around the section of the vein containing the catheter (Figure 3). Additional sutures can be placed to stabilize and secure the vein-catheter complex (for example to the periosteum or the skin of the forehead with a hang-back suture) to prevent inadvertent dislodgement of the catheter during the embolization portion of the procedure.
Once the vein has been cannulated and the catheter is capped and secured, then the case is passed to the neuro-interventional radiologist:
6. The guidewire and microcatheter is introduced through the catheter into the SOV under fluoroscopic guidance.
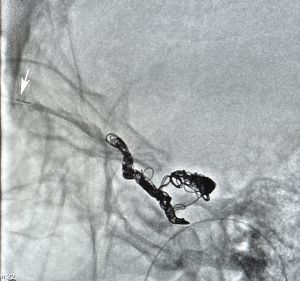
7. The cavernous sinus is successfully accessed, the guidewire is removed and the embolization device (balloon, coil, etc.) is positioned through the microcatheter. The neuro-interventional radiologist performs angiography and then the embolization. Final angiography is done to confirm cessation of flow through the fistula.
Following successful embolization the case is turned back to the orbital surgeon:
8. The SOV may be tied-off with 4-0 silk sutures and the catheter is then removed. Alternatively the ligature sutures may be loosened and then cautery may be used at the venipuncture site. Once hemostasis is confirmed, then the 2-0 silk ligature sutures are removed.
The orbital septum is left open, however the skin incision is closed according to surgeon’s preference.[21] Absorbable 6-0 plain gut suture in an interrupted fashion allows any post-operative bleeding an egress anteriorly and may prevent hemorrhage from accumulating in the retrobulbar space.
Surgical Follow-up, Complications, and Prognosis
The patient should be seen on post-operative day 1 to check vision, intraocular pressure, pupil and extraocular motility. Longer term follow up to monitor for post-operative infection, glaucoma and recurrence of CCF signs/symptoms should also be undertaken. Patients have described low-grade headache, dizziness, and nausea in the immediate postoperative period. This is believed to be secondary to low-grade aseptic meningitis following manipulation of the cavernous sinus. These symptoms have resolved spontaneously in all reported patients. Standard wound care must be applied to the lid-crease incision. However, with adequate care, the wound heals to an imperceptible scar. Postoperatively, patients should have close follow-up to ensure resolution of symptoms and lack of complications. However, reported cases describe positive outcomes without complications, demonstrating the safety of the procedure.[13][21][27]
Though uncommon, complications following direct canulation of the SOV can be severe, especially considering the vascular nature of the lesion, and the location of the fistula within the brain. These include infection of the cavernous sinus, orbit, or other orbital structures, rupture of the SOV and subsequent intraorbital hemorrhage, and surgical damage to nearby structures.[7][21] It is important to remember that transvenous embolization is accompanied by inherent risks, including migration of the embolization device and iatrogenic dissection or thrombosis.[2][20][23][24]
Outcomes in reported patients treated via direct canulation of the SOV are positive, with timely resolution of CCF symptoms and lack of adverse events.[13][21][27] This is especially notable when considering that most of the patients selected for this treatment were not amenable to, or had previously failed, standard transarterial or standard transvenous approaches to endovascular embolization. Additional work is necessary to fully understand the outcome of this procedure in a larger population.
Summary of Reported Cases
| Report | Number of patients | Rationale for SOV canulation | Complications | Outcomes |
| Teng et al., 1988[26] | 5 | 2 anatomically incompatible, 1 failed prior surgical attempt, 1 failed prior transarterial attempt, 1 failed prior transfemoral attempt | 1 with eyelid hematoma | All patients experienced complete resolution of ocular symptoms |
| Hanneken et al., 1989[13] | 4 | 2 anatomically incompatible, 2 failed prior transarterial attempts | None reported | All patients experience complete resolution of symptoms |
| Monsein et al., 1991[27] | 4 | 4 failed prior transarterial attempts | None reported | All patients experienced complete resolution of symptoms |
| Miller et al., 1995[28] | 12 | Insufficient data | 1 patient required postoperative embolization of residual fistula, 1 patient required postoperative surgical correction of CN6 paresis | All patients experienced complete resolution of symptoms |
| Goldberg et al., 1996[21] | 10 | 1 anatomically incompatible, 9 failed prior transarterial attempts | 1 with foreign body granuloma of the upper eyelid | 8 with complete resolution of symptoms, 1 with residual visual field deficit and ptosis, 1 lost to follow-up after 1 month |
| Quiñones et al., 1997[29] | 12 | 8 anatomically incompatible, 4 failed prior transarterial attempts | 1 with lid granuloma | 11 with complete resolution of symptoms and closure of fistula |
| Baldauf et al., 2004[30] | 2 | 2 failed prior endovascular attempts | None reported | Both patients experienced complete resolution of symptoms |
| Wolfe et al., 2010[36] | 10 | 10 failed prior endovascular attempts | None reported | 1 patient experienced only partial embolization, all experienced complete resolution of symptoms |
| Chalouhi et al., 2012[32] | 10 | 10 failed prior endovascular attempts | 1 with intractable high intraocular pressure | All patients experienced complete resolution of symptoms |
| Briganti et al., 2013[33] | 30 | Insufficient data | 1 with intractable high intraocular pressure, 1 with thrombophlebitis | 7 with residual ptosis, 1 with residual diplopia, 1 with persistent ptosis, visual impairment and corneal damage, 21 with complete resolution of symptoms |
| Jiang et al., 2013[34] | 9 | 9 with failed prior endovascular attempts | None reported | All patients experienced complete resolution of symptoms |
| El-Hindy et al., 2014[35] | 3 | 3 anatomically incompatible with endovascular attempt | 1 with temporary lid lagophthalmos secondary to wound contracture | 2 experienced complete resolution of symptoms, 1 had to be stopped due to canulation failure |
References
- ↑ Jump up to: 1.0 1.1 Laplant J, Nerva JD, Wong G, Scullen T, Zeoli T, Al-Zubidi N, et al. Carotid Cavernous Fistula. EyeWiki 2023. https://eyewiki.aao.org/Carotid_Cavernous_Fistula (accessed December 10, 2023).
- ↑ Jump up to: 2.0 2.1 2.2 2.3 2.4 Miller NR. Diagnosis and management of dural carotid-cavernous sinus fistulas. Neurosurg Focus 2007;23:E13. https://doi.org/10.3171/FOC-07/11/E13.
- ↑ Santos D dos, Monsignore LM, Nakiri GS, Cruz AAV e, Colli BO, Abud DG. Diagnóstico por imagem das fístulas arteriovenosas da região do seio cavernoso. Radiol Bras 2014;47:251–5. https://doi.org/10.1590/0100-3984.2013.1799.
- ↑ Debrun GM. Angiographic workup of a carotid cavernous sinus fistula (CCF) or what information does the interventionalist need for treatment? Surg Neurol 1995;44:75–9. https://doi.org/10.1016/0090-3019(95)00162-X.
- ↑ Rao QA, Newhouse JH. Risk of nephropathy after intravenous administration of contrast material: A critical literature analysis. Radiology 2006;239:392–7. https://doi.org/10.1148/radiol.2392050413.
- ↑ Azzam D, Cypen S, Tao J. Anatomy, Head and Neck: Eye Ophthalmic Vein. 2023.
- ↑ Jump up to: 7.0 7.1 Shah A, Patel BC. Superior Ophthalmic Vein Cannulation for Carotid Cavernous Fistula. 2023.
- ↑ Barrow DL, Spector RH, Braun IF, Landman JA, Tindall SC, Tindall GT. Classification and treatment of spontaneous carotid cavernous sinus fistulas. J Neurosurg 1985;62:248–56. https://doi.org/10.3171/jns.1985.62.2.0248.
- ↑ Thomas AJ, Chua M, Fusco M, Ogilvy CS, Tubbs RS, Harrigan MR, et al. Proposal of venous drainage-based classification system for carotid cavernous fistulae with validity assessment in a multicenter cohort. Neurosurgery 2015;77:380–5. https://doi.org/10.1227/NEU.0000000000000829.
- ↑ Alam MS, Jain M, Mukherjee B, Sharma T, Halbe S, Jaisankar D, et al. Visual impairment in high flow and low flow carotid cavernous fistula. Sci Rep 2019;9:1–7. https://doi.org/10.1038/s41598-019-49342-3.
- ↑ De Keizer RJW. Carotid-cavernous and orbital arteriovenous fistulas: Ocular features, diagnostic and hemodynamic considerations in relation to visual impairment and morbidity. Orbit 2003;22:121–42. https://doi.org/10.1076/orbi.22.2.121.14315.
- ↑ Ishijima K, Kashiwagi K, Nakano K, Shibuya T, Tsumura T, Tsukahara S. Ocular manifestations and prognosis of secondary glaucoma in patients with carotid-cavernous fistula. Jpn J Ophthalmol 2003;47:603–8. https://doi.org/10.1016/j.jjo.2003.08.002.
- ↑ Jump up to: 13.0 13.1 13.2 13.3 13.4 13.5 Hanneken AM, Miller NR, Debrun GM, Nauta HJW. Treatment of Carotid-Cavernous Sinus Fistulas Using a Detachable Balloon Catheter Through the Superior Ophthalmic Vein. Arch Ophthalmol 1989;107:87–92. https://doi.org/10.1001/archopht.1989.01070010089033.
- ↑ Jump up to: 14.0 14.1 Wang W, Li YD, Li MH, Tan HQ, Gu BX, Wang J, et al. Endovascular treatment of post-traumatic direct carotid-cavernous fistulas: A single-center experience. J Clin Neurosci 2011;18:24–8. https://doi.org/10.1016/j.jocn.2010.06.008.
- ↑ Pashapour A, Mohammadian R, Salehpour F, Sharifipour E, Mansourizade R, Mahdavifard A, et al. Long-term endovascular treatment outcome of 46 patients with cavernous sinus dural arteriovenous fistulas presenting with ophthalmic symptoms: A non-controlled trial with clinical and angiographic follow-up. Neuroradiol J 2014;27:461–70. https://doi.org/10.15274/NRJ-2014-10079.
- ↑ Marques MCP, Pereira Caldas JGM, Nalli DR, Fonseca JRF, Nogueira RG, Abdala N. Follow-up of endovascular treatment of direct carotid-cavernous fistulas. Neuroradiology 2010;52:1127–33. https://doi.org/10.1007/s00234-010-0683-8.
- ↑ Ringer AJ, Salud L, Tomsick TA. Carotid cavernous fistulas: Anatomy, classification, and treatment. Neurosurg Clin N Am 2005;16:279–95. https://doi.org/10.1016/j.nec.2004.08.004.
- ↑ Gemmete JJ, Chaudhary N, Pandey A, Ansari S. Treatment of carotid cavernous fistulas. Curr Treat Options Neurol 2010;12:43–53. https://doi.org/10.1007/s11940-009-0051-3.
- ↑ Kirsch M, Henkes H, Liebig T, Weber W, Esser J, Golik S, et al. Endovascular management of dural carotid-cavernous sinus fistulas in 141 patients. Neuroradiology 2006;48:486–90. https://doi.org/10.1007/s00234-006-0089-9.
- ↑ Jump up to: 20.0 20.1 20.2 Oishi H, Arai H, Sato K, Iizuka Y. Complications associated with transvenous embolisation of cavernous dural arteriovenous fistula. Acta Neurochir (Wien) 1999;141:1265–71. https://doi.org/10.1007/s007010050429.
- ↑ Jump up to: 21.0 21.1 21.2 21.3 21.4 21.5 21.6 21.7 Goldberg RA, Goldey SH, Duckwiler G, Vinuela F. Management of cavernous sinus-dural fistulas. Indications and techniques for primary embolization via the superior ophthalmic vein. Arch Ophthalmol (Chicago, Ill 1960) 1996;114:707–14. https://doi.org/10.1001/archopht.1996.01100130699011.
- ↑ Roy D, Raymond J. The role of transvenous embolization in the treatment of intracranial dural arteriovenous fistulas. Neurosurgery 1997;40:1133–41; discussion 1141-4. https://doi.org/10.1097/00006123-199706000-00004.
- ↑ Jump up to: 23.0 23.1 Meyers PM, Halbach V V, Dowd CF, Lempert TE, Malek AM, Phatouros CC, et al. Dural carotid cavernous fistula: definitive endovascular management and long-term follow-up. Am J Ophthalmol 2002;134:85–92. https://doi.org/10.1016/s0002-9394(02)01515-5.
- ↑ Jump up to: 24.0 24.1 Kim MS, Han DH, Kwon OK, Oh CW, Han MH. Clinical characteristics of dural arteriovenous fistula. J Clin Neurosci 2002;9:147–55. https://doi.org/10.1054/jocn.2001.1029.
- ↑ Isamat F, Ferrer E, Twose J. Direct intracavernous obliteration of high-flow carotid-cavernous fistulas. J Neurosurg 1986;65:770–5. https://doi.org/10.3171/jns.1986.65.6.0770.
- ↑ Jump up to: 26.0 26.1 Teng MMH, Guo WY, Huang CI, Wu CC, Chang T. Occlusion of arteriovenous malformations of the cavernous sinus via the superior ophthalmic vein. Am J Neuroradiol 1988;9:539–46.
- ↑ Jump up to: 27.0 27.1 27.2 27.3 27.4 Monsein LH, Debrun GM, Miller NR, Nauta HJW, Chazaly JR. Treatment of dural carotid-cavernous fistulas via the superior ophthalmic vein. Am J Neuroradiol 1991;12:435–9.
- ↑ Jump up to: 28.0 28.1 Miller NR, Monsein LH, Debrun GM, Tamargo RJ, Nauta HJ. Treatment of carotid-cavernous sinus fistulas using a superior ophthalmic vein approach. J Neurosurg 1995;83:838–42. https://doi.org/10.3171/jns.1995.83.5.0838.
- ↑ Jump up to: 29.0 29.1 Quiñones D, Duckwiler G, Gobin PY, Goldberg RA, Viñuela F. Embolization of dural cavernous fistulas via superior ophthalmic vein approach. AJNR Am J Neuroradiol 1997;18:921–8.
- ↑ Jump up to: 30.0 30.1 Baldauf J, Spuler A, Hoch HH, Molsen HP, Kiwit JC, Synowitz M. Embolization of indirect carotid-cavernous sinus fistulas using the superior ophthalmic vein approach. Acta Neurol Scand 2004;110:200–4. https://doi.org/10.1111/j.1600-0404.2004.00314.x.
- ↑ Wolfe GI, Kaminski HJ, Aban IB, Minisman G, Kuo H-C, Marx A, et al. Randomized Trial of Thymectomy in Myasthenia Gravis. N Engl J Med 2016;375:511–22. https://doi.org/10.1056/nejmoa1602489.
- ↑ Jump up to: 32.0 32.1 Chalouhi N, Dumont AS, Tjoumakaris S, Gonzalez LF, Bilyk JR, Randazzo CR, et al. The superior ophthalmic vein approach for the treatment of carotid-cavernous fistulas: A novel technique using Onyx. Neurosurg Focus 2012;32:1–6. https://doi.org/10.3171/2012.1.FOCUS123.
- ↑ Jump up to: 33.0 33.1 Briganti F, Caranci F, Leone G, Napoli M, Cicala D, Briganti G, et al. Endovascular occlusion of dural cavernous fistulas through a superior ophthalmic vein approach. Neuroradiol J 2013;26:565–72. https://doi.org/10.1177/197140091302600510.
- ↑ Jump up to: 34.0 34.1 Jiang C, Lv X, Li Y, Wu Z, Shi J. Surgical access on the superior ophthalmic vein to the cavernous sinus dural fistula for embolization. J Neurointerv Surg 2013;5:2011–4. https://doi.org/10.1136/neurintsurg-2011-010227.
- ↑ Jump up to: 35.0 35.1 El-Hindy N, Kalantzis G, Patankar T, Georgalas I, Jyothi S, Goddard T, et al. Difficult indirect carotid-cavernous fistulas-alternative techniques to gaining access for treatment. Clin Interv Aging 2014;9:1687–90. https://doi.org/10.2147/CIA.S69920.
- ↑ Wolfe SQ, Cumberbatch NMA, Aziz-Sultan MA, Tummala R, Morcos JJ. Operative approach via the superior ophthalmic vein for the endovascular treatment of carotid cavernous fistulas that fail traditional endovascular access. Neurosurgery 2010;66:293–9. https://doi.org/10.1227/01.neu.0000369705.91485.38.