Spectral Domain Optical Coherence Tomography in Glaucoma
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
Purpose
This is a review of the utility of spectral-domain optical coherence tomography in the diagnosis and management of glaucoma. It will provide a brief review of all modalities, but focus primarily on the Cirrus machine.
Introduction
Optical coherence tomography (OCT), first described in 1991, is a noncontact, noninvasive imaging technique that can reveal layers of the retina by looking at the interference patterns of reflected laser light.[1] Automated software segmentation algorithms are able to outline the retinal nerve fiber layer with much precision, which is relevant in glaucoma since this layer is thinned as ganglion cells are lost. OCT became widely popular in 2002 with the release of Stratus OCT, a time-domain technology (TD-OCT) that was well-studied and validated for use in glaucoma and retina and went on to become a standard structural imaging test. Only four years later, several companies started to release the next generation technology, spectral-domain OCT (SD-OCT), AKA fourier-domain OCT, which improved upon TD-OCT by capturing more data in less time at a higher axial image resolution, around 5 µm. Ultrahigh speed swept source OCT, ultrahigh resolution OCT, polarization sensitive OCT, and adaptive optics OCT are all on the horizon.
Currently, the most common four commercially available SD-OCT devices in the US are: Cirrus HD-OCT (Carl Zeiss Meditec, Dublin, CA, USA), RTVue-100 (Optovue Inc., Fremont, CA, USA), Spectralis OCT (Heidelberg Engineering, Heidelberg, Germany), and Topcon 3D-OCT 2000 (Topcon Corporation, Tokyo, Japan).[2] Each machine has different glaucoma scan patterns, proprietary software segmentation algorithms, and display outputs. Our review focuses on the Cirrus HD-OCT in diagnosing glaucoma and glaucoma progression.
SD-OCT Parameters
There are three main parameters relevant to the detection of glaucomatous loss: retinal nerve fiber layer, optic nerve head, and the “ganglion cell complex.” The numeric values for all parameters are shaded as white, green, yellow, or red, with the yellow and red representing, < 5% and < 1%, respectively compared to the normative database.
Retinal Nerve Fiber Layer
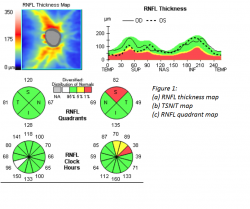
Glaucoma is a group of many conditions sharing a final common pathway characterized by accelerated death of retinal ganglion cells and their retinal nerve fiber layer (RNFL) axons resulting in characteristic visual field defects and corresponding optic nerve head anatomical changes. SD-OCT can directly measure and quantify RNFL thickness by calculating the area between the internal limiting membrane (ILM) and RNFL border (how the edge of the RNFL is determined and how blood vessels are handled are different among the machines, which do not have interchangeable measurement outputs).[3] The Cirrus RNFL map represents a 6 x 6 mm cube of A-scan data centered over the optic nerve in which a 3.4 mm diameter circle of RNFL data is extracted to create what is referred to as the TSNIT map (temporal, superior, nasal, inferior, temporal). It is displayed as a false color scale with the thickness values referenced to a normative database. The TSNIT map displays RNFL thickness values by quadrants and clock hours, and the RNFL peaks give a sense of the anatomic distribution of nerve fiber axons represented by the superior and inferior bundles that emanate from the optic nerve (see figure 1).1
Optic Nerve Head (ONH)
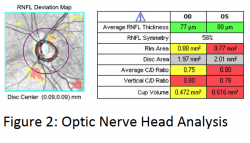
SD-OCT also automatically outlines the optic nerve head, optic cup, and disc borders similar to mental estimations by clinicians, but then also calculates more objective measurements such as optic disc area and neuroretinal rim area in addition to the classic clinician-subjective average and vertical cup-to-disc ratios. Cirrus does this by defining the edge of the disc as the termination of Bruch’s membrane and then finds the shortest perpendicular distance to ILM, AKA minimum band distance, to define the inner cup margin in each slice in a spiral around the optic disc cube data until a center is located. This allows the 3.4 mm RNFL circle to always be centered in the same spot within the cube, which was not possible with TD-OCT. The calculated ONH parameters, except for disc area, are then compared to a normative database (see figure 2).1
Ganglion Cell Analysis
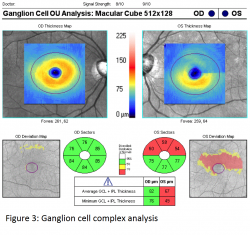
The ganglion cell layer is thickest in the perimacular region and decreased total macular thickness has been observed in glaucomatous eyes likely due to thinning of the ganglion cell layer in this region. However segmenting the ganglion cell layer alone is very difficult based on reflectivity and thus Cirrus chose to measure its Ganglion Cell Analysis (GCA) consisting of the combined ganglion cell layer (GCL) and inner plexiform layer (IPL). Optovue’s Ganglion Cell Complex (GCC) chose to include RNFL in their combined GCL and IPL layers. The Cirrus macular scan map (see figure 3) is displayed using a similar color scale and divided into various pie sectors around the fovea. Again the calculated sectors are compared to a normative database.1
Use of SD-OCT in Diagnosis of Glaucoma
Several studies have been performed to assess the diagnostic capability of SD-OCT in perimetric glaucoma. One representative study compared the diagnostic capability of SD-OCT to TD-OCT RNFL thickness scans in subjects with early and moderate glaucoma as well as normal age-matched subjects. When using the average RNFL thickness at the 5% level compared to the normative database (yellow coloring on RNFL deviation map), SD-OCT had a sensitivity of 83% and a specificity of 88% compared to 80% and 94% respectively for TD-OCT. When using the average RNFL thickness at the 1% level (red coloring on RNFL deviation map), the specificity for both SD-OCT and TD-OCT was 100% but the sensitivity was only 65% in SD-OCT and 61% in TD-OCT.[4]
ONH parameters have also been found to have excellent ability to discriminate between normal eyes and eyes with even mild glaucoma. The parameters found to have the greatest diagnostic capability are vertical rim thickness, rim area, and vertical cup to disc ratio. These ONH parameters were found to be as good as RNFL thickness parameters in diagnosing glaucoma.[5]
Similarly, GCA parameters have been found to be comparable to ONH and RNFL parameters. The parameters found to be most diagnostically useful are minimum, inferotemporal sector, average, superotemporal sector, and inferior sector ganglion cell complex thickness.[6]
It is well known that significant structural RNFL loss occurs prior to the development of functional visual field loss.[7] In such preperimetric disease, SD-OCT RNFL is especially useful in helping to diagnose glaucoma prior to the onset of visual field loss. In the presence of perimetric disease, finding RNFL bundle loss on SD-OCT with a corresponding abnormality in the visual field served by those retinal ganglion cells can help confirm the diagnosis of glaucoma.
Use of SD-OCT in Detection of Glaucomatous Progression
Repeatability and Reproducibility of SD-OCT
Studies have evaluated the reproducibility and repeatability of SD-OCT measures relevant to assessing change over time. One study of Cirrus found excellent intravisit and intervisit reproducibility of measurements of peripapillary RNFL thickness and ONH parameters. The intervisit tolerance limit for average RNFL thickness was 3.89 µm suggesting that a reproducible decrease of 4 µm or more may be a statistically significant change. [8] A more cautious cutoff would be a 10 µm change, roughly twice the maximum standard deviation found in that study. However, it is important to exclude any scans that are not of adequate quality. Scans with a signal strength less than 6, with eye movement or blinking artifacts within the 1.73-mm radius around the optic nerve head, or with segmentation errors should be repeated.
Trend and Event Analysis
Glaucoma progression algorithms can be divided into event-based and trend-based approaches, similar to visual field progression detection methods. Event-based analysis defines progressive change when a follow up measurement exceeds a pre-established threshold for change from baseline. Alternatively, trend-based analysis defines progression by monitoring the change over time using regression analysis to provide a rate of progression and corresponding significance level.2
Guided Progression Analysis (GPA)
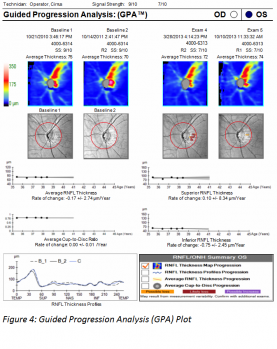
GPA, introduced in 2009 on the Cirrus, compares the RNFL thickness of individual clusters of A-scans, referred to as pixels, between baseline and follow up RNFL thickness maps to estimated test-retest variability. Local pixels exceeding such test-retest variability are coded in yellow at the first event, and in red if the same changes are seen on three consecutive images (see figure 4). In order to generate an overall trend plot, two baseline scans with three follow up scans are necessary. This linear regression line in µm/yr, representing rate of change, is drawn with an estimated confidence interval carried forward.
Progressive RNFL Thinning
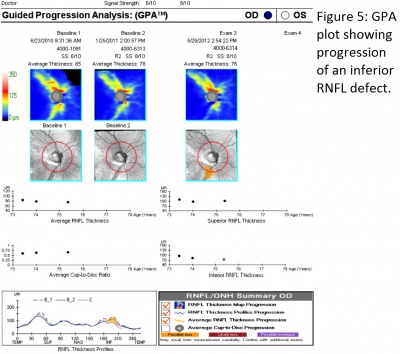
Progressive RNFL thinning measured on SD-OCT can often be used to detect progressive disease (see figure 5). The top three RNFL progression patterns are: widening of an existing RNFL defect, deepening without widening of an existing RNFL defect, or development of a new RNFL defect. In one study, the inferotemporal quadrant was the most frequent location for RNFL progression.[9]
Floor Effect
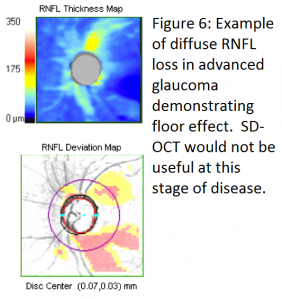
In early to moderate glaucoma, progressive thinning of RNFL thickness measured by SD-OCT is a very useful tool to judge progression of disease. At advanced stages however, SD-OCT is less clinically useful due to a “floor effect” of RNFL thickness. With advanced loss, RNFL thickness levels off, rarely falling below 50 µm and almost never below 40 µm due to the assumed presence of residual glial or nonneural tissue including blood vessels.[10] At this level of disease, serial visual fields are more useful to judge progression. (see figure 6)
Correlation of OCT with Visual Field
Due to the variability or possible artifacts with SD-OCT measurements, all changes on OCT should be correlated with visual field changes before confirming definite progression. When such correspondence is not found, caution should be exercised and sources of erroneous measurements should be sought, a few of which are reviewed below.
Sources of Misinterpretation
Normative Database
As previously stated, SD-OCT measurements are compared against an age-matched normative database. The normative database for the Cirrus SD-OCT consisted on 284 healthy individuals with an age range between 18 and 84 years (mean of 46.5 years). Ethnically, 43% were Caucasian, 24% were Asians, 18% were African American, 12% were Hispanic, 1% were Indian, and 6% were of mixed ethnicity. The refractive error ranged from -12.00 D to +8.00 D.3 Due to this relatively small normative database and wide variation of distribution of RNFL, many results obtained by SD-OCT may be flagged as abnormal statistically in patients who are not represented in the database and thus not necessarily representing real disease. Clinicians should use caution to avoid overtreating “red disease” in these situations.[11]
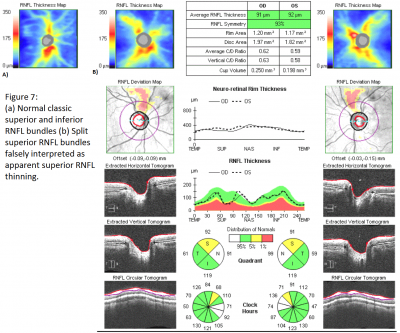
One common special case is the myopic patient. As mentioned previously, high myopes were not included in the normative database. Myopic eyes have thinner RNFL measurements, which can confound comparisons to the normative database. Additionally, myopic eyes can have unique distributions of RNFL bundles. With increasing myopia, the superotemporal and inferotemporal RNFL bundles tend to converge temporally.[12] This may result in the temporal shift of the superior and inferior RNFL bundle peaks of normal magnitude. Due to this shift, although the peaks are of normal magnitude, they can be interpreted as thinned due to having a different distribution from the normative database. Additionally, even in normal eyes, split RNFL bundles, both superiorly and inferiorly, have been found on histologic section, thus representing a true normal variant which may appear abnormal on SD-OCT (see figure 7).[13]
While the limitations of the normative database may hinder the utility of SD-OCT in diagnosing glaucoma using a single scan, serial SD-OCT scans can be very useful to judge glaucomatous progression by setting a baseline scan against which to judge progressive thinning on subsequent scans. Therefore, each patient can be his or her own “normative database” to diagnose glaucoma in such difficult settings as high myopia. With this approach, clinicians should be aware that RNFL thickness decreases with age in normal, healthy individuals. Based on a longitudinal study, the age-related rate of reduction in RNFL thickness has been estimated to be -0.52 µm/year, -1.35 µm/year, and -1.25 µm/year for average, superior, and inferior RNFL respectively.[14]
Signal Quality
When assessing the adequacy of a scan, the signal strength should always be noted. The signal strength, reported on a scale of 0 to 10, is defined as the averaged intensity value of the signal pixels in the OCT image. The best quality scans have signal strength greater than 8 (minimum acceptable scan > 6). In one study, artificially defocusing an image scan by +2 diopters resulted in an artifactual 10 µm thinning of the RNFL.[15] Similarly, a 9.3% increase in mean average RNFL thickness was seen after cataract surgery in a study of 45 patients.[16]
Blink/Saccades
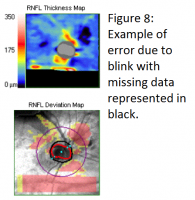
An SD-OCT RNFL scan consists of multiple single A-scans side by side to represent a B-scan cube. With eye movement or blinking, these scans do not align correctly which can lead to an erroneous RNFL thickness measurement, which may be misinterpreted as progressive thinning (see figure 8). The new SD-OCT versions have a built-in eye tracking function which can help compensate for eye movement by relying on blood vessel registration or iris tracking. Using the eye tracker significantly improves the reproducibility of RNFL measurements.[17]
Segmentation Errors
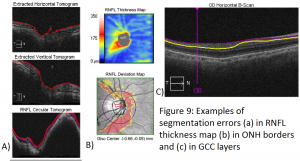
It is important to look at the segmentation lines produced by any SD-OCT machine’s software algorithm to ensure that they are appropriately placed. Lines should not come together (go to zero). Occasionally, one will find that the segmentation lines are misplaced along the retina leading to errors in the calculation of RNFL thickness (see figure 9). These segmentation errors are more common in the presence of poor signal strength, tilted discs, staphylomas, large peripapillary atrophy, epiretinal membranes, and posterior vitreous detachments. Studies have found a decreased incidence of such segmentation errors in SD-OCT compared to TD-OCT.[18]
Media Opacities
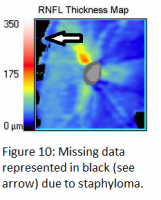
The en face scanning laser ophthalmoscope (SLO) image should be examined to ensure the absence of a media opacity, such as a posterior vitreous detachment (PVD), within the circumpapipillary scan area. Areas in which data is missing due to an opacity are represented in black on the en face SLO image (see figure 10). In such a case, the overlying PVD can lead to a falsely thin RNFL measurement in the underlying area. Additionally, as mentioned previously, cataracts can affect RNFL thickness measurements. One study found a 4.8 µm increase in RNFL thickness measurement after cataract surgery. This effect was most pronounced in cortical cataracts, followed by posterior subcapsular cataracts. Interestingly, nuclear cataracts were not found to affect signal strength or RNFL thickness measurements.[19]
Axial Length
Axial length has been shown to influence SD-OCT measurements of both RNFL thickness and ONH parameters due to axial-length induced ocular magnification. The longer the eye, the thinner the RNFL, and the smaller the optic disc area and neuroretinal rim area.[20] Axial-length induced ocular magnification is not currently accounted for by Cirrus SD-OCT. However, refractive error independent of axial length has not been found to affect RNFL thickness measurements as long as a well focused fundus image is obtained during scan acquisition by utilizing the Cirrus SD-OCT internal fixation focus adjustment.[21] This adjustment can account for refractive errors from -20.0 D to +20.0 D.[22]
Summary
SD-OCT is a powerful objective structural assessment tool that can greatly assist clinicians in diagnosing and managing glaucoma (especially early disease), when used in conjunction with visual field testing and serial clinical exams.
References
- ↑ Aref AA, Budenz DL. Spectral domain optical coherence tomography in the diagnosis and management of glaucoma. Ophthalmic Surg Lasers Imaging 2010;41:S15-27
- ↑ Grewal DS, Tanna AP. Diagnosis of glaucoma and detection of glaucoma progression using spectral domain optical coherence tomography. Curr Opin Ophthalmol 2013;24:150-161.
- ↑ Leite MT, Rao HL, Weinreb RN, et al. Agreement among spectral-domain optical coherence tomography instruments for assessing retinal nerve fiber layer thickness. Am J Ophthalmlol 2011;151:85-92.
- ↑ Chang RT, Knight OJ, Feuer WJ, Budenz DL. Sensitivity and specificity of time-domain vs spectral-domain optical coherence tomography in diagnosing early to moderate glaucoma. Ophthalmology 2009; 116:1257-1263.
- ↑ Mwanza JC, Oakley JD, Budenz DL, Anderson DR. Ability of Cirrus HD-OCT Optic Nerve Head Parameters to Discriminate Normal from Glaucomatous Eyes. Ophthalmology 2011;118:241-248.
- ↑ Mwanza JC, Durbin MK, Budenz DL, et al. Glaucoma diagnostic accuracy of ganglion cell-inner plexiform layer thickness: comparison with nerve fiber layer and optic nerve head. Ophthalmology 2012;119:1151-1158.
- ↑ Quigley HA, Dunkelberger GR, Green WR. Retinal ganglion cell atrophy correlated with automated perimetry in human eyes with glaucoma. Am J Ophthalmol. 1989;107(5):453–64.
- ↑ Mwanza JC, Chang RT, Budenz DL, et al. Reproducibility of peripapillary retinal nerve fiber layer thickness and optic nerve head parameters measured with cirrus HD-OCT in glaucomatous eyes. Invest Ophthalmol Vis Sci 2010;51:5724-5730.
- ↑ Leung CK, Yu M, Weinreb RN, et al. Retinal nerve fiber layer imaging with spectral-domain optical coherence tomography: patterns of retinal nerve fiber layer progression. Ophthalmology 2012;119:1858-1866.
- ↑ Hood D, Kardon R. A framework for comparing structural and functional measurements of glaucomatous damage. Prog Retin Eye Res 2007;26:688-710.
- ↑ Chong GT, Lee RK. Glaucoma versus red disease: imaging and glaucoma diagnosis. Curr Opin Ophthalmol 2012;23:79-88.
- ↑ Leung CK, Yu M, Weinreb RN, et al. Retinal nerve fiber layer imaging with spectral-domain optical coherence tomography: interpreting the RNFL maps in healthy myopic eyes. Invest Ophthalmol Vis Sci 2012;53:7194-7200.
- ↑ Kaliner E, Cohen MJ, Miron H, et al. Retinal nerve fiber layer split bundles are true anatomic variants. Ophthalmology 2007;114:2259-2264.
- ↑ Leung CK, Yu M, Weinreb RN, et al. Retinal nerve fiber layer imaging with spectral-domain optical coherence tomography: a prospective analysis of age-related loss. Ophthalmology 2012;119:731-737.
- ↑ Balasubramanian M, Bowd C, Vizzeri G, et al. Effect of image quality on tissue thickness measurements with spectral-domain optical coherence tomography. Opt Express 2009;17:4019-4036.
- ↑ Mwanza JC, Bhorade AM, Sekhon N, et al. Effect of cataract and its removal on signal strength and peripapillary retinal nerve fiber layer optical coherence tomography measurements. J Glaucoma 2011;20:37-43.
- ↑ Langenegger SJ, Funk J, Toteberg-Harms M. Reproducibilty of retinal nerve fiber layer thickness measurements using the eye tracker and the retest function of Spectralis SD-OCT in glaucomatous and healthy control eyes. Invest Ophthalmol Vis Sci 2011;52:3338-3344.
- ↑ Ho J, Sull AC, Vuong LN, et al. Assessment of artifacts and reproducibility across spectral- and time-domain optical coherence tomography devices. Ophthalmology. 2009;116:1960–1970.
- ↑ Lee DW, Kim JM, Park KH. Effect of media opacity on retinal nerve fiber layer thickness measurements by optical coherence tomography. J Ophthalmol Vis Res 2010;5:151-157.
- ↑ Savini G, Barboni P, Parisis V, et al. The influence of axial length on retinal nerve fibre layer thickness and optic-disc size measurements by spectral-domain OCT. Br J Ophthalmol 2012;96:57-61.
- ↑ Sharma N, Sony P, Gupta A, et al. Effect of laser in situ keratomileusis and laser-assisted subepithelial keratectomy on retinal nerve fiber layer thickness. J Cataract Refract Surg 2006;32:446-450.
- ↑ Carl Zeiss Meditec “Cirrus HD-OCT 5000 and 500: Technical Specifications” (http://applications.zeiss.com/C1257A290053AE30/0/4BEACDCC7BCD1769C1257B6000392EB0/$FILE/CIRRUS_5000_Tech_Spec_CIR.4769.pdf). Accessed November 30, 2013.