Schwartz-Jampel Syndrome
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
Original contributors: Jaber J, Chandra K, Langston W, Huang A, Yen MT
Disease Entity
Schwartz-Jampel Syndrome (SJS), otherwise known as chondrodystrophic myotonia, is a genetic disorder that leads to muscle and bone deformities during development. Specifically, it leads to stiffness and weakness, shorter stature, and a “mask-like” expression associated with blepharophimosis. In regards to further eye-related symptoms, affected individuals may also be nearsighted and experience blepharospasm. [1]
Disease Background
There are historically two types of SJS: Type 1 (classified into subtypes A and B) and Type 2. Type 1A is the classic presentation of SJS, where onset occurs later in life with mild symptoms compared to Type 1B. Type 2 is now considered a distinct and more severe condition called Stuve-Wiedemann Syndrome and will not be covered in this article. [2]
Anatomy
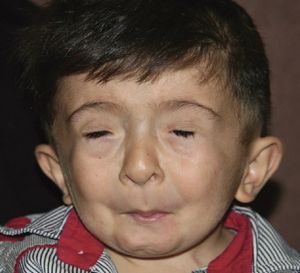
Schwartz-Jampel Syndrome is a systemic disease and thus affects multiple parts of the body. In the context of eye disease, the most common symptom is blepharophimosis. Blepharophimosis affects the normal anatomy of the eye with narrow eye openings, droopy eyelids, and folds of skin near the inner eye. The characteristic mask-like appearance of the periocular muscles of Schwartz-Jampel Syndrome can be attributed to underlying myotonia, contractions, and blepharospasms due to the loss of perlecan and its ability to retain proper neuromuscular junction interactions. Because of the persistent contraction of the orbicularis muscle, growth of the eyelids are diminished and result in the phenotypic form of blepharophimosis. Further, the levator tone is compromised by the sustained orbicularis spasm, but has been noted to usually remain functionally intact. Due to potential stiffness of extraocular eye muscles, patients can also present with diplopia and strabismus. Presentation of blepharophimosis in a child and adult patient with SJS can be seen below: [4] [5] [6]
Etiology
SJS is a rare genetic disorder with only about 150 cases that have been reported in medical literature. [1]
Inheritance and Causes
SJS is an autosomal recessive disorder that is caused by a mutation in the perlecan gene, HSPG2, which is located on chromosome 1p36.1-p35.[7] Perlecan is a component of the basement membrane, and mutations in the sulfate proteoglycan can result in SJS and severe neonatal lethal dyssegmental dysplasia Silverman-Handmaker (DDSH) type.
According to one study, there are at least five different mutations in the perlecan gene that have been documented to cause SJS. In all these mutations, the SJS perlecan gene still creates partially functional perlecan that can be used within extracellular matrices. The study noted that from the three patients sequenced, one had heterozygous mutations in the gene and all patients had varying presentation and onset of symptoms. Onset and recognition of symptoms ranged from birth to 3 months to 4 years. [8]

In a detailed analysis, there were many more mutation types found to be associated with SJS and DDSH. Mutations in the perlecan gene are rare and have effects that range from mild to severe or perinatally lethal. Complete loss-of-function mutations of the HSP2G gene are lethal. However, in studies using mouse models, if one allele of the perlecan gene is present, SJS phenotype is observed and the amount of perlecan is reduced by >90%. [9]
General Pathology / Symptoms
The cardinal symptoms of SJS are skeletal dysplasia and neuromuscular activity. Specifically, to the eyes, presentation of SJS may include blepharophimosis, intraocular lens subluxation, poor visual acuity, and ectopia lentis. Furthermore, pupillary configuration, trace light reflex, and dilation in response to mydriatics can be abnormal or less exaggerated than expected.
The above symptoms are associated with the loss of perlecan. Perlecan plays a key role in the cross-linking of extracellular matrix components to help maintain muscle bulk homeostasis, acetylcholinesterase content at the end motor endplate, excitability at the nerve terminal, and the muscle membrane. Specifically, perlecan exists in the extracellular matrix of endothelial tissue, cartilage, bone marrow, and muscle and is an ubiquitous component of the muscle membrane. It directly retains acetylcholinesterase levels, and deficiency of perlecan will lead to instability in the neuromuscular junction. Therefore, the loss of perlecan directly leads to a loss of acetylcholinesterase, leading to motor endplate hyperactivity.
The perlecan protein has five domains, with domains II and V being involved with calcium signaling and sodium channels, respectively. Dysfunction of domain II can lead to impaired calcium signaling, which in turn affects calcium dynamics though calcium-ATPase 1 and ryanodine receptor 1 in the endoplasmic reticulum. The relationship between domain II and the calcium signaling further affects muscle contraction. Further, domain II plays a key role in perlecan’s stability. Mutations in perlecan can impact disulfide bonds found in domain II, which ultimately leads to low levels of perlecan expression from ubiquitination and degradation of the protein.
Other facial symptoms include muscle cramping, proximal muscle hypertrophy, retruded jaw, malocclusion, extruded jaw and dimorphism. It is important to note that there are variable mutations in the HSPG2 gene, and the different variations may lead to different severity, onset, or expression of the SJS phenotype. [7]
Bone abnormalities of SJS syndrome can include the following: Joint deformities and limitations in motion, coxa valga, irregularity of the capital femoral epiphysis, kyphosis, short neck, and pectus carinatum. [10]
Histologic Findings
No specific electron microscope is known for the disorder; however, there are minor ultrastructural abnormalities that have been described. Light microscope findings suggest myopathy and variations in the size of muscle fibers are common. With age and disease advancement, fat and connective tissue replacement of muscles may occur. [10]
Diagnosis
History
Infants with type 1 SJS have a history of low to normal birth weight with slower than normal growth rate beginning in the first or second year of life. Most infants with SJS are delayed in reaching motor development milestones such as crawling, sitting, or walking. Adult height may be below the normal range; however, mental development is typically normal. Type 2 SJS is typically diagnosed at birth.[11][12]
Physical examination
Principle characteristics upon general physical exam include myotonic myopathy, dystrophy of epiphyseal cartilages, joint contractures, below average stature, low and posteriorly rotated pinnae, and pectus carinatum. Joint contractures often involve wrists, elbows, and shoulders. Additionally, the shoulders tend to be shifted anteriorly and internally rotated. [11]
Common ocular presentations upon exam include blepharophimosis, blepharospasm, microphthalmia, and microcornea In addition, patients with SJS may present with myopia and early-onset cataracts. The extent of visual impairment varies per individual. For instance, severe blepharospasm can result in blindness.[2][13] Additional presentations of SJS can include a closed mouth with pursed lips, extra rows of eyelashes, continuous contraction of facial muscles, a high-arched palate, and a high-pitched nasally voice. [11][14]
Diagnostic procedures
SJS can be prenatally diagnosed via ultrasonography.[15] However, SJS is typically diagnosed at birth or within the first three years of life. Myotonic myopathy, a principle characteristic of the disorder, can be identified through electromyography (EMG).[14] Other methods of confirming myotonic myopathy include biopsy of muscle tissue and subsequent examination under light or electron microscopy or checking for elevated serum creatine kinase or aldolase. Skeletal abnormalities can be visualized and confirmed via imaging studies. [16] Genetic testing can subsequently be used to confirm diagnosis.
Differential diagnosis
- Stiff person syndrome
- Isaac's syndrome
- Congenital myotonic dystrophy
- Myotonic dystrophy
- Myotonia congenita
- Congenital muscular dystrophy
- Morquio's syndrome
- Ehlers-Danlos syndrome
- Malignant hyperthermia
- Blepharospasm
- Stuve-Wiedemann syndrome
Management
Medical therapy
Medical therapy is primarily aimed at symptomatic treatment and prevention of progression of SJS. Muscle relaxants and anti-epileptic drugs may alleviate myotonia and some studies have shown that early treatment can delay progression and lessen debilitating effects. Carbamazepine, procainamide, mexiletine, and phenytoin have been shown to be useful in managing myotonia. [17] Botulinum toxin A has been tested as an therapeutic option, but has shown mixed results.[18] Physical therapy can be indicated to prevent formation of joint contractures and improve skeletal abnormalities. [11]
Surgery therapy
Surgical treatment for Schwartz-Jampel Syndrome is aimed at improvement of cosmetic and functional abnormalities. Patients with SJS experience ptosis (drooping of the upper eyelid), blepharophimosis (abnormally small palpebral fissures), and blepharospasm (uncontrolled squeezing of the eyelids). Treatment options include increasing the width and height of the palpebral fissure, as well as relieving the blepharospasm. Surgeries targeted at these outcomes include orbicularis oculi myectomy, resection and advancement of the levator aponeurosis, and lateral canthopexy. [19]
Orbicularis Oculi Myectomy
The orbicularis oculi are paired ocular muscles that function to close the eyelids. In patients with SJS, continuous myotonia of the orbicularis oculi leads to the development of blepharospasm.[20] Blepharospasm is the abnormal contraction of the eyelids and can lead to an inability to open the eyelids in patients with SJS. Orbicularis oculi myectomy involves the removal of muscle fibers from around the eye and is performed to reduce the blepharospasm associated with SJS.[3] The orbicularis oculi muscle is divided into orbital and palpebral sections. The palpebral section can be further subdivided into preseptal and pretarsal sections. The preseptal and pretarsal sections of the muscle are responsible for involuntary closure of the eyelids, and therefore a target in the treatment of SJS-associated blepharospasm.[21] In the orbicularis oculi myectomy procedure for treating blepharospasm, muscle fibers are removed from the preseptal and pretarsal portions of eyelids. [22] This procedure can be done on only the upper eyelids, or both the upper and lower eyelids. By removing muscle fibers from the orbicularis oculi, overall muscle tone is diminished and blepharospasm is corrected.
Resection and Advancement of Levator Aponeurosis
The levator palpebrae superioris muscle is another paired ocular muscle that functions to elevate the upper eyelid. In patients with SJS, the function of this muscle is normal, but it is overcome by the continuous myotonia of the orbicularis oculi muscle.[22] The increased tone of the orbicularis oculi results in stretched and weakened levator palpebrae superioris muscles, drooping eyelids (ptosis), and abnormally small palpebral fissures. Resection and advancement of this muscle aponeurosis are performed with the goal of increasing the palpebral fissure height in patients with SJS.[23] This surgery is performed by making an incision at the supratarsal fold, followed by dissection of the orbicularis oculi muscle to expose the tarsal plate. The anterior portion of the tarsal plate is then separated from the levator aponeurosis. The levator aponeurosis is then advanced and any excess tissue is removed. The depth of advancement during this surgery varies depending on the severity of the ptosis and blepharophimosis. Finally, the levator aponeurosis is sutured to the superior tarsal plate in its advanced position.[24] By advancing the aponeurosis of the levator palpebrae superioris to a more posterior portion of the superior tarsal plate, the tension of the muscle is increased and ptosis is corrected.
Lateral Canthopexy
The lateral canthal tendon connects the upper and lower tarsal plates to Whitnall’s tubercle on the lateral orbital wall. The orbicularis oculi muscle has attachments to both the medial and lateral canthal tendons.[25] In patients with SJS and increased orbicularis oculi muscle tone, the lateral canthal tendon may experience reduced tension. The increased laxity of the lateral canthal tendon contributes to the blepharophimosis seen in patients with SJS.[23] Surgical treatment involves the attachment of the retinaculum of the lateral canthal tendon to the periosteum of the lateral orbital wall with a suture.[26] By strengthening the attachment of the tendon to the orbital wall, the width of the palpebral fissure is increased. This aids in correcting blepharophimosis associated with SJS.
Complications
Patients with SJS have classically been considered to be at higher risk of malignant hyperthermia. Avoidance of triggering agents (volatile anesthetic agents or succinylcholine) is generally recommended when anesthetizing patients with SJS to prevent the occurrence of malignant hyperthermia. Due to the increased risk, patients with SJS should undergo surgical therapies only after failing to respond to medical therapies. Malignant hyperthermia is potentially lethal and SJS patients that undergo general anesthesia should be closely monitored. Dantrolene should always be available to treat this possible complication.[27]
Possible complications following orbicularis oculi myectomy include loss of sensation, dry eyes, skin loss requiring a graft, hematoma, hemorrhage, eyelash loss, brow hair loss, canthal deformity and trichiasis.[28] Despite these possibilities, complications associated with orbicularis oculi myectomy are rare.[29]The most common complication associated with levator aponeurosis advancement is undercorrection, which may require additional surgery. Other complications include overcorrection, hematoma, and infection.[24]
Summary
In summary, Schwartz-Jampel Syndrome is an autosomal recessive disorder that presents with myopathy, bone deformities, and facial/orbital abnormalities due to the loss of the perlecan. Common eye presentations of SJS include blepharophimosis and blepharospasm, along with visual deficits. Diagnosis of SJS typically occurs within the first three years of life due to delayed motor developments. Common tests for SJS include ultrasonography, EMG, and blood tests; however, diagnoses can be confirmed through genetic testing. Medical therapy for SJS is aimed at symptomatic treatment and prevention of the progression of SJS. In comparison, surgical management of the syndrome is aimed at improving cosmetic and functional abnormalities.
References
- ↑ 1.0 1.1 U.S. National Library of Medicine. (n.d.). Schwartz-Jampel Syndrome: Medlineplus genetics. MedlinePlus [Internet]. Retrieved April 5, 2023, from https://medlineplus.gov/genetics/condition/schwartz-jampel-syndrome/#resources
- ↑ 2.0 2.1 U.S. Department of Health and Human Services. (n.d.). Schwartz Jampel syndrome - about the disease. Genetic and Rare Diseases Information Center. Retrieved April 5, 2023, from https://rarediseases.info.nih.gov/diseases/250/schwartz-jampel-syndrome
- ↑ 3.0 3.1 Eshraghi, B., Shadravan, M., Aalami, E., & Pour, E. K. (2016). Orbicularis Oculi Myectomy as a Treatment for Blepharospasm in a Case of Schwartz Jampel Syndrome. Journal of Ophthalmic & Vision Research, 11(3), 329–332. https://doi.org/10.4103/2008-322X.188401
- ↑ Ding, J., Xu, Y., Yuan, B., & Li, D. (2021). Management of blepharospasm and blepharophimosis associated with Schwartz-Jampel syndrome. Journal of AAPOS: The Official Publication of the American Association for Pediatric Ophthalmology and Strabismus, 25(1), 54–56. https://doi.org/10.1016/j.jaapos.2020.10.009
- ↑ Doshi, H., Cheng, T., Barmettler, A., Alsuhaibani, A., & Burkat, C. N. (n.d.). Blepharophimosis syndrome. EyeWiki. Retrieved April 19, 2023, from https://eyewiki.aao.org/Blepharophimosis_Syndrome#cite_note-beaconsfield24-2
- ↑ Blepharophimosis Syndrome. (n.d.). Cleveland Clinic. Retrieved April 26, 2023, from https://my.clevelandclinic.org/health/diseases/24529-blepharophimosis-ptosis-epicanthus-inversus-bpes
- ↑ 7.0 7.1 7.2 Lin, P.-Y., Hung, J.-H., Hsu, C.-K., Chang, Y.-T., & Sun, Y.-T. (2021). A Novel Pathogenic HSPG2 Mutation in Schwartz-Jampel Syndrome. Frontiers in Neurology, 12, 632336. https://doi.org/10.3389/fneur.2021.632336
- ↑ Arikawa-Hirasawa, E., Le, A. H., Nishino, I., Nonaka, I., Ho, N. C., Francomano, C. A., Govindraj, P., Hassell, J. R., Devaney, J. M., Spranger, J., Stevenson, R. E., Iannaccone, S., Dalakas, M. C., & Yamada, Y. (2002). Structural and Functional Mutations of the Perlecan Gene Cause Schwartz-Jampel Syndrome, with Myotonic Myopathy and Chondrodysplasia. American Journal of Human Genetics, 70(5), 1368–1375.
- ↑ Martinez, J. R., Dhawan, A., & Farach-Carson, M. C. (2018). Modular Proteoglycan Perlecan/HSPG2: Mutations, Phenotypes, and Functions. Genes, 9(11), Article 11. https://doi.org/10.3390/genes9110556
- ↑ 10.0 10.1 Kubrey, S., Solanki, D., & Agrawal, S. (2015). Schwartz-Jampel Syndrome (SJS) a rare entity: Case report. Journal of Evolution of Medical and Dental Sciences, 4(43), 7538–7547.
- ↑ 11.0 11.1 11.2 11.3 Pascuzzi, R. M. (1991). Schwartz-Jampel syndrome. Seminars in Neurology, 11(3), 267–273. https://doi.org/10.1055/s-2008-1041231
- ↑ Schwartz Jampel Syndrome—Symptoms, Causes, Treatment | NORD. (n.d.). Retrieved April 26, 2023, from https://rarediseases.org/rare-diseases/schwartz-jampel-syndrome/
- ↑ Aberfeld, D. C., Namba, T., Vye, M. V., & Grob, D. (1970). Chondrodystrophic myotonia: Report of two cases. Myotonia, dwarfism, diffuse bone disease, and unusual ocular and facial abnormalities. Archives of Neurology, 22(5), 455–462. https://doi.org/10.1001/archneur.1970.00480230073009
- ↑ 14.0 14.1 Basiri, K., Fatehi, F., & Katirji, B. (2015). The Schwartz-Jampel syndrome: Case report and review of literature. Advanced Biomedical Research, 4, 163. https://doi.org/10.4103/2277-9175.162538
- ↑ Hunziker, U. A., Savoldelli, G., Boltshauser, E., Giedion, A., & Schinzel, A. (1989). Prenatal diagnosis of Schwartz-Jampel syndrome with early manifestation. Prenatal Diagnosis, 9(2), 127–131. https://doi.org/10.1002/pd.1970090208
- ↑ Spaans, F., Theunissen, P., Reekers, A. D., Smit, L., & Veldman, H. (1990). Schwartz-Jampel syndrome: I. Clinical, electromyographic, and histologic studies. Muscle & Nerve, 13(6), 516–527. https://doi.org/10.1002/mus.880130608
- ↑ Topaloğlu, H., Serdaroğlu, A., Okan, M., Gücüyener, K., & Topçu, M. (1993). Improvement of myotonia with carbamazepine in three cases with the Schwartz-Jampel syndrome. Neuropediatrics, 24(4), 232–234. https://doi.org/10.1055/s-2008-1071547
- ↑ Aburahma, S. K., Al-Khateeb, T., Alrefai, A., & Amarin, Z. (2009). Botulinum toxin A injections for the treatment of Schwartz-Jampel syndrome: A case series. Journal of Child Neurology, 24(1), 5–8. https://doi.org/10.1177/0883073808320621
- ↑ Lucci, L. M., Yen, M. T., Anderson, R. L., Hwang, I. P., & Black, R. E. (2001). Orbicularis myectomy with levator advancement in schwartz-jampel syndrome. American Journal of Ophthalmology, 132(5), 799–801. https://doi.org/10.1016/S0002-9394(01)01104-7
- ↑ Ozge, G., Uysal, Y., Melih Ceylan, O., & Erdurman, F. C. (2015). Surgical management of 2 cases with Schwartz-Jampel syndrome. Canadian Journal of Ophthalmology, 50(6), e110–e112. https://doi.org/10.1016/j.jcjo.2015.08.012
- ↑ Tong J, Lopez MJ, Patel BC. Anatomy, Head and Neck: Eye Orbicularis Oculi Muscle. [Updated 2022 Jul 25]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK441907/
- ↑ 22.0 22.1 Morrison, D. A., Mellington, F. B., Hamada, S., & Moore, A. T. (2006). Schwartz-Jampel Syndrome: Surgical Management of the Myotonia-Induced Blepharospasm and Acquired Ptosis After Failure With Botulinum Toxin A Injections: Ophthalmic Plastic & Reconstructive Surgery, 22(1), 57–59. https://doi.org/10.1097/01.iop.0000195008.15872.a0
- ↑ 23.0 23.1 Gillum, W. N. (1981). Blepharospasm Surgery: An Anatomical Approach. Archives of Ophthalmology, 99(6), 1056. https://doi.org/10.1001/archopht.1981.03930011056015
- ↑ 24.0 24.1 Harvey, D. J., Iamphongsai, S., & Gosain, A. K. (2010). Unilateral Congenital Blepharoptosis Repair by Anterior Levator Advancement and Resection: An Educational Review: Plastic and Reconstructive Surgery, 126(4), 1325–1331. https://doi.org/10.1097/PRS.0b013e3181ebe1e9
- ↑ Rosenstein, T., Talebzadeh, N., & Pogrel, M. A. (2000). Anatomy of the lateral canthal tendon. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology, and Endodontology, 89(1), 24–28. https://doi.org/10.1016/S1079-2104(00)80009-8
- ↑ Rizvi, M., Lypka, M., Gaon, M., Eisemann, B., Eisemann, M., & Lypka, M. (2010). A Simplified Lateral Canthopexy Technique: Plastic and Reconstructive Surgery, 125(6), 248e–249e. https://doi.org/10.1097/PRS.0b013e3181d45d19
- ↑ de Oliveira Camacho, F., Lopes Amaral, T., & de Barros Mourão, J. (2018). A successful anesthetic approach in a patient with Schwartz–Jampel syndrome. Saudi Journal of Anaesthesia, 12(1), 128. https://doi.org/10.4103/sja.SJA_393_17
- ↑ Pariseau, B., Worley, M. W., & Anderson, R. L. (2013). Myectomy for blepharospasm 2013: Current Opinion in Ophthalmology, 1. https://doi.org/10.1097/ICU.0b013e3283645aee
- ↑ Dinjar, K. (2020). Surgical Aspect of Blepharospasm Treatment: A Case Report. Acta Clinica Croatica. https://doi.org/10.20471/acc.2020.59.02.25

