Sanjad-Sakati Syndrome
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
Disease Entity
Sanjad-Sakati syndrome (SSS), also known as hypoparathyroidism-retardation-dysmorphism syndrome (OMIM #241410), is a rare autosomal recessive disorder seen primarily in those with genetic ties to the Arabian Peninsula. Sanjad et al. reported the first case of SSS in 1988, followed by a case series of patients with SSS in 1991.[1] Primary manifestations of SSS include congenital hypoparathyroidism, intrauterine growth restriction, developmental delay, seizures, and facial dysmorphism. Ocular manifestations have also been reported, including phenotypically small eyes, corneal opacities, optic nerve abnormalities, and retinal abnormalities. Understanding the ocular manifestations of SSS is crucial for accurate diagnosis, appropriate management, and improved patient outcomes.
Epidemiology
SSS predominantly affects children with genetic ties to the Arabian Peninsula and is more prevalent in offspring from consanguineous relationships. Incidence rates of 7-18 per 100,000 live births in Kuwait have been reported, with some Middle Eastern ethnic groups having as high an incidence as 1 per 5,000 live births.[2][3]
Genetics
SSS is an autosomal recessive disorder caused by a 12-base pair deletion in the tubulin-specific chaperone E gene (TBCE) located on the long arm of chromosome 1 (1q42-43).[4] Tubulin-specific chaperone proteins are required for the proper folding of α-tubulin subunits and the formation of β-tubulin heterodimers. Tubulins are essential components of multiple cellular structures, including cilia, flagella, and mitotic spindles.[5] The ocular abnormalities seen in SSS are likely a result of disrupted embryonic development due to faulty microtubule assembly.
Diagnosis
Systemic Manifestations
Primary manifestations of SSS include congenital hypoparathyroidism, intrauterine growth restriction, developmental delay, and facial dysmorphism (Table 1). Primary hypoparathyroidism is the main endocrinological manifestation of SSS. Affected patients may present with hypo-calcemic tetany, apnea, seizures, hyperphosphatemia, physical and mental growth delays, and craniofacial and orodental deformities within the first few weeks of life. The classic craniofacial features described in the literature include microcephaly, deep-set eyes, beaked nose, depressed nasal bridge, long philtrum, thin lips, micrognathia, and thick large floppy ear lobes. Additional endocrinological features reported in patients with SSS include growth hormone deficiency and hypothyroidism.[1],[6],[7] Reports of recurrent infections from functional hyposplenism have also been reported; bacterial infection is the most common cause of death in SSS patients.[7]
| Systemic Features | Ocular Features |
|---|---|
|
|
Ocular Manifestations
Ophthalmological manifestations in SSS are diverse and can involve the anterior and posterior segments of the eye (Table 2). The most common ocular manifestation was phenotypically small eyes, including microphthalmia, microcornea, and nanophthalmos (34 patients, 52 eyes), followed by strabismus (23 patients), retinal vascular tortuosity (21 patients, 42 eyes), and corneal opacities including band keratopathy (Figure 1), corneal pannus, corneal stromal edema, and corneal opacity not otherwise specified (16 patients, 20 eyes). Other less common features include optic nerve involvement, including optic nerve swelling, optic nerve atrophy, and optic nerve abnormalities not otherwise specified (12 patients, 7 eyes), cataracts (6 patients, at least 2 eyes), nystagmus (5 patients), retinal detachment (4 patients, 1 eye), retinopathy (3 patients), persistent fetal vasculature (1 patient, 1 eye), and childhood glaucoma (1 patient, 2 eyes).[1],[2],[5],[8],[9],[10], [11], [12] See Figure 2.
The severity and combination of these manifestations vary widely among affected individuals.
| Study | Number of Patients | Number of Males:Females | Average Age (years) | Number of Patients with: Number of patients (Number of eyes) | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Phenotypically Small Eyes | Strabismus | ONS | ONA | Optic Nerve Abnormalities | Nystagmus | Coloboma | RVT | RD | Retinopathy | PFV | Cataract | Childhood Glaucoma | Corneal Opacity | Band Keratopathy | CSE | Pannus | ||||||
| Microphthalmia | Nanopthalmos | Microcornea | ||||||||||||||||||||
| Al Dhoyan et al.5 | 17 | 10:7 | 5.47 | 3 (6) | 17 (34) | 11 (na) | 3 (3) | 17 (34) | 3 (na) | |||||||||||||
| David et al.2 | 25 | 10:15 | 6.2 | 2 (na) | 6 (na) | 1 (na) | 1 (na) | 3 (na) | 1 (na) | 1 (na) | 3 (na) | 3 (na) | 5 (na) | 2 (na) | ||||||||
| Elhusseiny and Saeed8 | 2 | 0:2 | 1.25 | 1 (2) | 1 (na) | 1 (2) | 2 (3) | 1 (2) | ||||||||||||||
| Haider et al.9 | 1 | 0:1 | 2 | 1 (2) | 1 (2) | 1 (2) | 1 (2) | 1 (1) | 1 (1) | 1 (2) | ||||||||||||
| Khan et al.10 | 4 | 2:2 | 2.69 | 4 (8) | 2 (4) | 4 (7) | 1 (2) | 1 (2) | ||||||||||||||
| Ryabets-Lienhard et al.11 | 1 | 0:1 | 4 | 1 (2) | ||||||||||||||||||
| Albaramki et al.12 | 3 | na | na | 1 (2) | 1 (2) | 1 (2) | ||||||||||||||||
| Sanjad et al.1 | 12 | 6:6 | 0.326 | 6 (na) | 5 (na) | 3 (na) | ||||||||||||||||
| Total | 65 | 28:34 | 4.36 | 10 (8) | 5 (10) | 19 (34) | 23 (na) | 6 (7) | 1 (na) | 3 (na) | 5 (2) | 1 (na) | 21 (42) | 4 (1) | 3 (na) | 1 (1) | 6 (2) | 1 (2) | 11 (11) | 3 (5) | 1 (2) | 1 (2) |
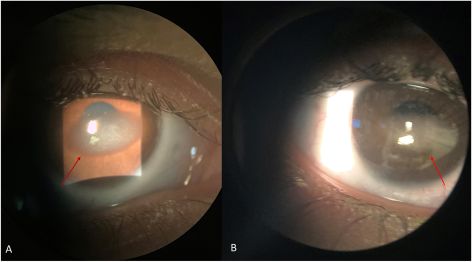
The original case series of SSS published by Sanjad et al.[1] described a group of 12 patients. The main ocular findings described in this report were microphthalmos (6 patients), strabismus (5 patients), and nystagmus (3 patients).
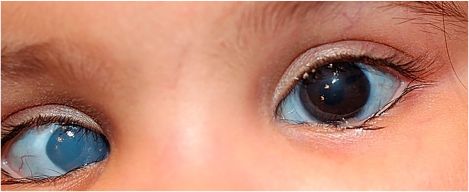
In the largest survey of patients with SSS, David et al.[2] reported a series of 63 patients who were seen at a single medical center. Of these patients, only 25 were referred for ophthalmological exams. Three of these patients had multiple anomalies. Microcornea (2 patients), strabismus (6 patients), optic nerve swelling (1 patient), optic nerve atrophy (1 patient), optic nerve abnormalities not otherwise specified (4 patients), nystagmus (1 patient), retinal detachment (3 patients), retinopathy not otherwise specified (3 patients), cataracts (5 patients), and corneal opacities not otherwise specified (2 patients) were reported in this sample. One patient in this group developed bilateral optic nerve swelling and was later diagnosed with pseudotumor cerebri after initiating growth hormone treatment for their systemic disease. The patient with optic nerve atrophy was diagnosed with septo-optic dysplasia.
In the second largest series, Al Dhoyan et al.[5] reported on the ophthalmological findings in a series of 17 patients, all seen at one of two medical centers in Saudi Arabia. All patients in this series underwent complete ophthalmic evaluations, including examinations under anesthesia. All patients in the series had bilateral microcorneas, with horizontal corneal diameter measurements ranging from 7.5 mm to 10.5 mm. Axial length was measured in three patients in the series aged four to 10 years, ranging from 15.93 mm to 17.8 mm. All patients in the series were found to have bilateral retinal vasculature tortuosity on fundus examination. In addition, unilateral optic nerve swelling was observed in three patients and was attributed to possible buried optic disc drusen by the authors. Optic nerve swelling due to buried optic disc drusen was also reported in a patient with SSS by Haider et al.[9] Albaramki et al.[12] reported one patient with papilledema but did not describe the underlying etiology.
Differential Diagnosis
Congenital hypoparathyroidism has several differentials, including SSS, familial hypoparathyroidism, Di-George syndrome, and Kenny-Caffey syndrome (KCS). Familial hypoparathyroidism lacks the characteristic dysmorphic features found in SSS.7 The clinical findings of SSS are reminiscent of KCS, which has two types: an autosomal dominant variant (type 2) characterized by the presence of osteosclerosis, normal intelligence, late closure of the anterior fontanelle, macrocephaly and postnatal growth retardation[5],[13] and an autosomal recessive variant (type 1) that shares many characteristics with SSS. Both conditions have been mapped to the same 12-base pair deletion in the 1q43-44 region.[4] Similar ocular manifestations have been found in patients with KSC Type 1 and Type 2.[13] KSC Type 1 is believed to be differentiated from SSS by the addition of osteosclerosis and recurrent bacterial infections. However, recurrent infections in SSS patients have also been reported.[4]
Management
Initial Examination and Laboratory Evaluation
Patients suspected of having SSS should undergo a thorough history and general physical assessment emphasizing detecting the classical dysmorphic facial features and clinical signs of hypoparathyroidism/hypocalcemia, including Chvostec sign, apnea, or seizures.
Patients should have genetic screening for the 12 bp deletion in the TCBE gene in the 1q43-44 region.
In primary hypoparathyroidism, decreased serum calcium and elevated serum phosphorus are expected. Absent or decreased serum parathyroid hormone (PTH) in conjunction with the above electrolyte abnormalities confirms primary hypoparathyroidism. Intracranial (particularly basal ganglia calcifications) can be seen on brain imaging by CT/MRI secondary to hyperphosphatemia.[7]
A complete blood count that is within normal ranges of blood components, especially T-cells, can help distinguish SSS from Di-George syndrome, a condition classically associated with craniofacial abnormalities, congenital hypoparathyroidism, thymus aplasia, and thus lack of T-cell immunity, and cardiac defects.[7]
Treatment
Multispecialty treatment, including genetics, endocrinology, and ophthalmology, should be instigated for all patients suspected of having SSS. Patients with SSS will require life-long treatment for primary hypoparathyroidism, which involves treating and managing their chronic hypocalcemia with chronic calcium and vitamin D supplementation. Consultation with an endocrinologist may be beneficial to aid in any comorbid growth hormone deficiencies or thyroid disorders. Genetic counseling should be offered to affected parents as prevention of future occurrences can be made through carrier detection and pre-implantation genetic diagnosis.
Prognosis Concerning Ocular Manifestations
Few data exist regarding the visual acuity (VA) of patients with SSS. Al Dhoyan et al.[5] reported that the VA of all 17 of their patients was normal, as indicated by central, steady, and maintained fixation. In one case series of two patients, Elhusseiny and Saeed[8] report VA of 20/125 in the right eye and 20/100 in the left eye in a 10-year-old female with bilateral microcornea and central band keratopathy. The second patient in this report, a 6-month-old female with bilateral childhood glaucoma with subepithelial corneal haze, corneal stromal edema, and a large left corneal calcium plaque, had a VA of poor fixation in the right eye and fix and follow in the left eye. Elhusseiny and Saeed[8] reported using EDTA chelation for corneal opacities in two patients.
In conclusion, SSS is a long-term, advancing condition in which treatment primarily focuses on providing relief and support. The cause of early death in most children with SSS is recurrent infections thought to be related to functional hyposplenism. Given the high rate of ocular involvement in patients with SSS syndrome, the authors posit that all patients suspected of having SSS should undergo at least an initial evaluation by an experienced pediatric ophthalmologist.
References
- ↑ Jump up to: 1.0 1.1 1.2 1.3 Sanjad SA, Sakati NA, Abu-Osba YK, Kaddoura R, Milner RD. A new syndrome of congenital hypoparathyroidism, severe growth failure, and dysmorphic features. Arch Dis Child. 1991;66(2):193-196.
- ↑ Jump up to: 2.0 2.1 2.2 David O, Agur R, Novoa R, et al. Hypoparathyroidism-retardation-dysmorphism syndrome-Clinical insights from a large longitudinal cohort in a single medical center. Front Pediatr. 2022;10:916679. doi:10.3389/fped.2022.916679
- ↑ Naguib KK, Gouda SA, Elshafey A, et al. Sanjad-Sakati syndrome/Kenny-Caffey syndrome type 1: a study of 21 cases in Kuwait. East Mediterr Health J Rev Sante Mediterr Orient Al-Majallah Al-Sihhiyah Li-Sharq Al-Mutawassit. 2009;15(2):345-352.
- ↑ Jump up to: 4.0 4.1 4.2 Parvari R, Hershkovitz E, Grossman N, et al. Mutation of TBCE causes hypoparathyroidism– retardation–dysmorphism and autosomal recessive Kenny–Caffey syndrome. Nat Genet. 2002;32(3):448-452. doi:10.1038/ng1012
- ↑ Jump up to: 5.0 5.1 5.2 5.3 5.4 Al Dhoyan N, Al Hemidan AI, Ozand PT. Ophthalmic manifestations of Sanjad-Sakati syndrome. Genetics. 2006;27(3):83-87. doi:10.1080/13816810600862568
- ↑ Arabi WA, Basheer AA, Abdullah MA. Sanjad-Sakati Syndrome in Sudanese children. Sudan J Paediatr. 2011;11(1):42-47.
- ↑ Jump up to: 7.0 7.1 7.2 7.3 Bashar M, Taimur M, Amreek F, Sayeed KA, Tahir A. Endocrinological Manifestations of Sanjad-Sakati Syndrome. Cureus. 12(6):e8770. doi:10.7759/cureus.8770
- ↑ Jump up to: 8.0 8.1 8.2 Elhusseiny AM, Saeed HN. Corneal opacification in Sanjad-Sakati syndrome. Am J Case Rep. 2022;26((Elhusseiny A.M.; Saeed H.N., Hajirah_Saeed@meei.harvard.edu) Department of Ophthalmology, Boston Children’s Hospital, Harvard Medical School, Boston, MA, United States). doi:10.1016/j.ajoc.2022.101503
- ↑ Jump up to: 9.0 9.1 Haider AS, Ganesh A, Al-Kindi A, et al. New Ocular Associations in Sanjad-Sakati Syndrome: Case report from Oman. Sultan Qaboos Univ Med J. 2014;14(3):e401-404.
- ↑ Khan AO, Al-Assiri A, Al-Mesfer S. Ophthalmic features of hypoparathyroidism-retardation-dysmorphism. J AAPOS. 2007;11(3):288-290. doi:10.1016/j.jaapos.2006.10.015
- ↑ Ryabets-Lienhard A, Issaranggoon na Ayuthaya S, Graham JM, Pitukcheewanont P. A case of severe TBCE-negative hypoparathyroidism-retardation-dysmorphism syndrome: Case report and literature review. Am J Med Genet A. 2018;176(8):1768-1772. doi:10.1002/ajmg.a.38851
- ↑ Jump up to: 12.0 12.1 Albaramki J, Akl K, Al-Muhtaseb A, et al. Sanjad Sakati syndrome: a case series from Jordan. East Mediterr Health J Rev Sante Mediterr Orient Al-Majallah Al-Sihhiyah Li-Sharq Al-Mutawassit. 2012;18(5):527-531. doi:10.26719/2012.18.5.527
- ↑ Jump up to: 13.0 13.1 Sabry MA, Farag TI, Shaltout AA, et al. Kenny-Caffey syndrome: an Arab variant? Clin Genet. 1999;55(1):44-49. doi:10.1034/j.1399-0004.1999.550108.x

