Retrobulbar Shunt
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
The retrobulbar shunt is a silicone glaucoma device that connects aqueous humor from the anterior chamber to the retrobulbar space. The retrobulbar shunt is a useful device for lowering both IOP and the number of glaucoma medications in patients with previously failed glaucoma surgeries, including glaucoma drainage devices. The main advantage is its ability to decrease IOP markedly and predictably [1].
Another advantage is the low risk of complications and failures due to cicatrization because of the retrobulbar aqueous redirection. It eliminates the need for the formation of a bleb or placement of a reservoir plate, minimizing the risk of leaks and exposures, and strabismus and diplopia. It has demonstrated predictable results in challenging situations among eyes with failed blebs, eyes scarring from previous ocular surgery, and eyes with connective tissue disorders[1]. Its retrobulbar placement potentially reduces the risk of fibrotic sequestration of aqueous humor [2][3]. It does not use an anterior or traditional subconjunctival bleb or plate, and there is no “valve”.
Device
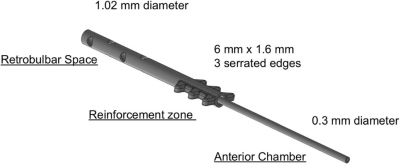
The tube has three segments: (a) anterior portion, placed into the anterior chamber, measuring 0.3 mm in diameter (the tube is identical in size to that is used in the Ahmed, Baerveldt, or Molteno implants); (b) reinforcement portion containing flanges that are used to fixate the tube at the sulcus level, measuring 6 × 1.6 mm in diameter; and (c) posterior portion introduced into the retrobulbar space (adipose tissue) that measures 1.02 mm in diameter and contains fenestrations for aqueous humor outflow [1].
Mechanism of action
The mechanism of action of the retrobulbar shunt relates the direction of aqueous humor into the retrobulbar space. The retrobulbar fat creates less scar tissue than those found in the Tenon’s capsule [2]. The microcanaliculi between the fat cells allows the aqueous humor to reach the hydrophilic periosteum of the orbit, thereby eliminating the need for a bleb. Therefore, shunting aqueous humor into the retrobulbar space [4] results in continuous aqueous circulation without a reservoir's need. Animal studies with retrobulbar shunt found no traces of fibrosis in the retrobulbar shunt [3]. The retrobulbar shunt is less likely to suffer from fibrotic encapsulation than traditional shunts implanted in the Tenon’s capsule. The retrobulbar space's intrinsic properties limit the risk of fibrosis, the principal cause of bleb failure[1].
Surgical technique
The retrobulbar shunt surgery takes approximately half the time required for standard shunt placement, avoids the placement of sutures over the retina, and eliminates bleb-related postoperative concerns. It is recommended a scleral graft to protect the tube[1].
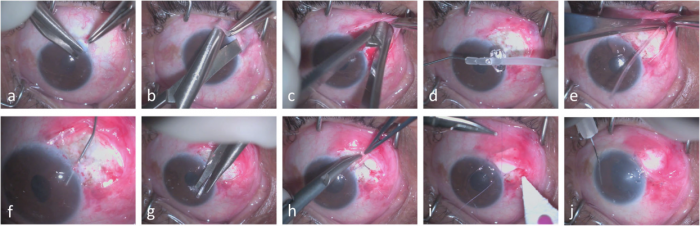
The surgery can be done under retrobulbar or subtenon anesthesia. The surgical steps include a fornix-based flap; opening the subconjunctival space to the retrobulbar space. The retrobulbar shunt is primed with viscoelastic through both sides to avoid the presence of air. The connection to the anterior chamber is accomplished using a 21- or 23-gauge needle to make a perforation 1.5 mm posterior to the limbus in line with the iris. The anterior portion of the tube is trimmed obliquely to the intracameral length and then introduced through the previous perforation. The shunt is fixated by applying 4 loops of 9-0 prolene around the tube. It is recommended a scleral graft over the tubing with 10-0 nylon. The Tenon’s capsule and the conjunctiva are closed with 8-0 Vicryl[1].
Results
The retrobulbar shunt was first clinically studied to communicate dysfunctional, fibrotic, and encapsulated tube shunt plates to the retrobulbar space, thereby returning them to normal function. 19 patients maintained substantial IOP reduction from a baseline of 33.3 ± 2.1 to 16.0 ± 1.6 at 24 months (P < 0.00001) [5]. The long-term results of the retrobulbar shunt in 35 eyes with fibrotic encapsulated blebs showed an IOP reduction from 30.94 ± 1.62 to 13.4 ± 1.23 at 5 years (n = 12; P < 0.0001) [6].
The retrobulbar shunt in patients with refractory glaucoma that have failed all other glaucoma surgeries and therapies is an effective rescue therapy. 19 eyes at 6 months dropped from baseline of 35.3 ± 2.3 to 18.5 ± 1.1(- 16.8, - 47%; p < 0.0001) 1. The mean number of glaucoma medications (±SEM) at 30, 90, and 180 days decreased from a baseline of 2.4 ± 0.3 to < 0.3 at each interval (p < 0.0002). The mean number of prior incisional glaucoma surgeries was 3.2, with 79% previously failed tube shunt surgeries[1].
Follow-up
The surgery includes a generous introduction of viscoelastic into the anterior chamber after implanting the device to prevent postoperative hypotony the next day. In the follow-up treatment with prednisolone acetate and moxifloxacin is administered 4 times a day for the first week, 3 times a day the second week, twice daily for the third week, and once daily during the fourth week before stopping it[1].
Safety
No patients experienced complications, loss of vision, shunt leaks, infections, or corneal edema. The main incidence is postoperative hypotony (IOPs < 5 mmHg) on day 1. It may appear if the eye has not been properly filled with viscoelastic during surgery; however, none of the eyes presented with hypotony related complications. In the consult, the anterior chamber can be reformed with viscoelastic resolving hypotony.
Discussion
Glaucoma surgery includes minimally invasive glaucoma surgery (MIGS), trabeculectomy non-penetrating and penetrating, antimetabolite filtering procedures, and ultimately glaucoma drainage devices (GDD) [7] as Ahmed, Baerveldt, and Molteno. The GDD use silicone tubes to shunt aqueous humor from the anterior chamber to a plate between the Tenon’s capsule and the subconjunctival space. These devices can fail for fibroblast-induced fibrosis of the episclera, Tenon’s capsule, and conjunctiva, resulting in encapsulation. This encapsulation diminishes the shunt’s ability to redirect aqueous humor out of the anterior chamber into the sub-Tenon microvasculature, causing IOP elevation, qualifying it as a failure [7][5][6]. Once shunts fail, patients are left with few therapeutic options, including placing a new shunt over a different quadrant of the eye or cyclophotocoagulation. The eyes in which one plate has become encapsulated are at high risk of the same outcome with subsequent surgeries.
From the pooled data of the ABC and AVB, approximately 49% of Ahmed shunts, and 37% of Baerveldt shunts fail within 5 years of implantation [8]. The usual options available to these patients include placing a traditional secondary shunt in a separate quadrant or performing cyclophotocoagulation. The postoperative course of sequential GDD implantation can be unpredictable because of the trauma already present from the original implant. Furthermore, implantation of a sequential GDD may further increase the risk of developing strabismus and diplopia [9][10]. Compared with the sequential GDD [11], the IOP reduction and percent IOP reduction is far greater with the retrobulbar shunt, while the reduction in glaucoma medications required is comparable. Retrobulbar shunt compared to the Ahmed Versus Baerveldt (AVB) and Ahmed Baerveldt Comparison (ABC) studies [12][13][14] obtains a greater mean reduction in IOP than primary Ahmed shunts, and comparable to the mean reduction achieved with primary Baerveldt shunts. Similarly, there is a greater reduction in mean glaucoma medications among these refractory retrobulbar shunts than those receiving primary Ahmed shunts, and comparable to the primary Baerveldt shunt. None of the subjects in the AVB or ABC studies had prior shunts, whereas 79% of those in the retrobulbar shunt had standard shunts that had failed before retrobulbar shunt implantation. The retrobulbar shunt performs better than GDDs in their primary surgery even when secondarily used after GDD failure[1].
References
- ↑ Jump up to: 1.0 1.1 1.2 1.3 1.4 1.5 1.6 1.7 1.8 Montelongo M, March de Ribot F, Craven ER, Sponsel WE. Retrobulbar Tube Shunt: Anterior Chamber to Back of the Eye (A2B) Efficacy in Glaucomatous Eyes with Uncontrolled IOP. Graefes Arch Clin Exp Ophthalmol. 2020 Nov 11.
- ↑ Jump up to: 2.0 2.1 Stahnke T, Löbler M, Kastner C et al (2012) Different fibroblast subpopulations of the eye: a therapeutic target to prevent postoperative fibrosis in glaucoma therapy. Exp Eye Res 100:88–97. https:// doi.org/10.1016/j.exer.2012.04.015
- ↑ Jump up to: 3.0 3.1 Batlle OR, Sponsel WE, Swann FB et al (2014) Retrobulbar diversion of aqueous humor. J Glaucoma 23(9):624–627. https://doi.org/ 10.1097/ijg.0000000000000077
- ↑ Kakizaki H, Takahashi Y, Nakano T et al (2012) Anatomy of Tenon’s capsule. Clin Exp Ophthalmol 40(6):611–616. https:// doi.org/10.1111/j.1442-9071.2011.02745.x
- ↑ Jump up to: 5.0 5.1 Sponsel WE, Groth SL, Ayyala RS (2014) Retrobulbar diversion of aqueous humor: clinical feasibility studies. J Glaucoma 23(9):628– 632. https://doi.org/10.1097/IJG.0000000000000082
- ↑ Jump up to: 6.0 6.1 Sponsel WE, Groth S, March de Ribot F et al (2019) Efficacy of a novel retrobulbar extension shunt to rescue eyes with fibrotic encapsulated blebs and uncontrolled ocular hypertension. Graefes Arch Clin Exp Ophthalmol 257(4):791–798. https://doi.org/10. 1007/s00417-018-4186-3
- ↑ Jump up to: 7.0 7.1 Prum BE, Rosenberg LF, Gedde SJ et al (2016) Primary OpenAngle Glaucoma Preferred Practice Pattern® guidelines. Ophthalmology 123(1):P41–P111. https://doi.org/10.1016/j. ophtha.2015.10.053
- ↑ Christakis PG, Zhang D, Budenz DL et al (2017) Five-Year Pooled Data Analysis of the Ahmed Baerveldt Comparison Study and the Ahmed Versus Baerveldt Study. Am J Ophthalmol 176:118–126. https://doi.org/10.1016/j.ajo.2017.01.003
- ↑ Islamaj E, Jordaan-Kuip CP, Vermeer KA et al (2018) Motility changes and diplopia after Baerveldt glaucoma drainage device implantation or after trabeculectomy. Transl Vis Sci Technol 7(5): 7. https://doi.org/10.1167/tvst.7.5.7
- ↑ Sun PY, Leske DA, Holmes JM, Khanna CL (2017) Diplopia in medically and surgically treated patients with glaucoma. Ophthalmology 124(2):257–262. https://doi.org/10.1016/j.ophtha. 2016.10.006
- ↑ Anand A, Tello C, Sidoti PA et al (2010) Sequential glaucoma implants in refractory glaucoma. Am J Ophthalmol 149(1):95– 101. https://doi.org/10.1016/j.ajo.2009.07.019
- ↑ Barton K, Gedde SJ, Budenz DL et al (2011) The Ahmed Baerveldt comparison study: methodology, baseline patient characteristics, and intraoperative complications. Ophthalmology 118(3):435– 442. https://doi.org/10.1016/j.ophtha.2010.07.015
- ↑ Budenz DL, Barton K, Gedde SJ et al (2015) Five-year treatment outcomes in the Ahmed Baerveldt comparison study. Ophthalmology 122(2):308–316. https://doi.org/10.1016/j.ophtha. 2014.08.043
- ↑ Christakis PG, Kalenak JW, Zurakowski D et al (2011) The Ahmed versus Baerveldt study: one-year treatment outcomes. Ophthalmology 120(11):2232–2240. https://doi.org/10.1016/j. ophtha.2011.05.004