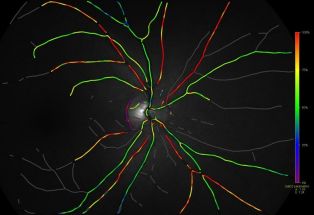
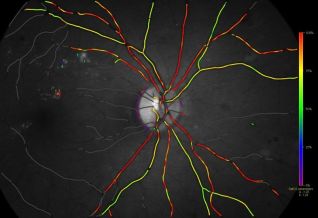
Retinal Oximetry
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
Definition and Background
The retina is one of the most metabolically active tissues in the human body, requiring a continuous supply of oxygen. Studies in primate models have shown that ischemia lasting more than 100 minutes leads to loss of retinal function and structural integrity.[1] Hypoxia plays a major role in various retinal vascular diseases, such as diabetic retinopathy and retinopathy of prematurity. Therefore, measuring oxygen levels in the living retina represents an important clinical challenge.
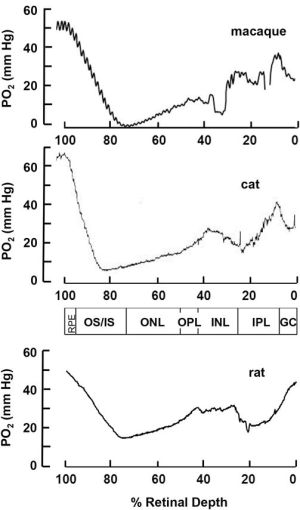
Retinal oximetry refers to technologies that can non-invasively measure oxygenation in retinal tissue, thus potentially providing markers of hypoxic stress in retinal tissue. Our understanding of retinal oxygenation from both retinal and choroidal circulations comes primarily from oxygen microelectrode experiments.[2] In these studies, microelectrodes are inserted into animal retinas, where the measured currents are proportional to the local oxygen pressure (pO2). This technique has provided detailed oxygen profiles throughout the retinal depth (Figure 1), establishing our fundamental understanding of retinal oxygen physiology.
Modern imaging technologies enable non-invasive retinal oximetry. These methods typically measure the oxygen saturation of hemoglobin (sO2) in retinal vessels rather than tissue pO2. In arterioles, approximately 97% of oxygen is bound to hemoglobin, with the remainder dissolved in plasma. The sO2 is defined as the ratio of the concentration of oxygenated hemoglobin to the total concentration of hemoglobin:
The relationship between sO2 and plasma pO2 follows a non-linear curve known as the oxyhemoglobin dissociation curve. All imaging-based oximetry methods rely on the different light absorption properties (extinction coefficients) of oxygenated and deoxygenated hemoglobin in the visible spectrum. The most studied method to perform retinal oximetry is based on multi-wavelength fundus photography, the use of which continues to evolve.[4] Newer approaches for retinal oximetry in patients include hyperspectral imaging,[5] visible-light optical coherence tomography,[6] and photoacoustic ophthalmoscopy.[7] This article will focus primarily on the non-invasive measurement technique using fundus photography.
Historical Development
The clinical use of oximetry began in 1935 when German physician Karl Matthes developed an ear O2 saturation meter. Retinal oximetry emerged in the 1950s, but recent advances in digital technology have expanded its clinical and research applications. Since 1963, multiple groups have developed non-invasive methods to measure retinal sO2. Currently, three commercial systems are available: the Oxymap Retinal Oximeter (Oxymap ehf, Iceland), the Vesselmap system (Imedos Systems GmbH, Germany), and the Zilia Ocular™ FC Retinal Camera (Zilia Inc, Canada). [8] [9] [10][11][12]

Technical Principles
The foundational principle of retinal oximetry relies on light absorption spectroscopy. Oxygenated and deoxygenated forms of hemoglobin exhibit distinct light absorption patterns across the visible spectrum. This difference manifests as the visible color variation between arteries and veins. By measuring the changes in light absorption, calculation of the relative ratio of the two species of hemoglobin can be performed, thus providing a value of the sO2.
Multi-wavelength fundus photography employs a fundus camera with an attached image splitter that divides the returning light spectrum into distinct, nearly monochromatic wavelength bands corresponding to specific points on the oxygen absorption curve. The Oxymap system, for example, uses a splitter that images at two distinct wavelengths: 600 nm (sensitive to oxyhemoglobin) and 570 nm (not sensitive to oxyhemoglobin). Optical density measurements are calculated using the equation OD=log(I/I0), where I represents image values within the vessel and I0 represents brightness values outside the vessel. The sO2 calculation follows the equation: SO2= a+k(ODx/ODy) where ODx and ODy are optical density measurements at wavelengths x and y respectively, and a and k are constants.
Limitations and Considerations
Several factors influence retinal oximetry measurements using dual-wavelength methods, including retinal and choroidal pigmentation, vessel size and thickness, linear blood flow velocity, and retinal nerve fiber layer thickness. These variables have been examined through both Monte Carlo simulations and experimental studies, and must be considered when interpreting clinical results.
Multivariate regression analyses of normative values in healthy subjects have revealed no significant associations between retinal oxygen saturation and iris pigmentation, ethnicity, gender, or smoking status. However, aging has been shown to decrease both retinal venous (SvO2) and arterial oxygen saturation (SaO2), making it an important factor to consider when interpreting and comparing measurements.[8][9][10][13]
Retinal Oximetry in Ocular Diseases
Retinal Vein Occlusions
Retinal vein occlusions occur when blood vessels in the retina become blocked, typically due to atherosclerosis and blood clot formation.[14] [15] These occlusions may be central, occurring near the optic nerve, or branch occlusions at arteriolar or venular bifurcations. Blood flow obstruction can lead to retinal ischemia in affected areas. Retinal oximetry shows promise in differentiating between ischemic and non-ischemic branch retinal vein occlusions (BRVO).
Research comparing arterial and venous oxygen saturation in patients with ischemic and non-ischemic BRVO demonstrated that patients with ischemic BRVO showed decreased venous oxygen saturation in affected vessels with increased arterial saturation. Conversely, in non-ischemic BRVO, the mean venous oxygen saturation was lower in the affected vein.[16] Other studies have shown more variable venous saturation patterns, with hypoxia present in some eyes but not others, potentially reflecting differences in disease severity and degree of occlusion.[17]
Studies of central retinal vein occlusion (CRVO) have linked venous oxygen saturation and arteriovenous difference to a retinal ischemic index measured by ultra-widefield fluorescein angiography.[18] This index is calculated by comparing the area of capillary nonperfusion to the total affected area, suggesting potential applications for monitoring retinal ischemic status through oximetry.
Diabetic Retinopathy
Diabetic retinopathy develops when chronic hyperglycemia damages retinal capillaries.[15][19] [20] Like retinal vascular occlusions, hypoxia plays a central role in disease progression. Retinal oximetry findings support theories of blood shunting and perfusion bypass in affected areas, leading to increased oxygenation in retinal venules.
Patients with retinopathy generally show higher venous oxygen saturation levels compared to healthy subjects, with a correlation between duration of diabetes and oxygen saturation.[21][22] This seemingly paradoxical finding likely results from poor oxygen distribution, increased total blood flow, or decreased oxygen consumption.[21] The increase in venous oxygenation correlates with disease severity.[23][24] Following laser photocoagulation treatment, arteriovenous saturation measurements indicate improved oxygen distribution.[25] As such, retinal oximetry has promising clinical applications for the diagnosis and management of the disease. (Figure 4A & 4B)
Studies using other types of oximetry systems have confirmed these findings. Studies using scanning laser ophthalmoscopy with Oxymap software have shown increased venous saturation in non-proliferative diabetic retinopathy, along with decreased arterial diameter and increased venous saturation in proliferative cases.[26] Hyperspectral computed tomographic imaging spectroscopy has revealed lower arteriovenous differences in patients with proliferative diabetic retinopathy compared to healthy controls.[27]
Glaucoma
Glaucoma is a chronic optic neuropathy with progressive injury to optic nerve axons and subsequent atrophy of retinal nerve fiber layer and ganglion cells.[15][28] [29] This atrophy is expected to reduce oxygen consumption in the inner retina. Consequently, venous oxygen saturation and arteriovenous saturation differences can offer insight into glaucomatous disease progression and provide new modalities of monitoring patients. Research also suggests that retinal vascular dysregulation might be part of the primary pathophysiology in certain types of glaucoma[21]. In addition to aiding in diagnosis and monitoring glaucoma, retinal oximetry might therefore offer new insight into an incompletely understood disease entity.
A 2014 Oxymap system study found positive correlations between glaucomatous damage (assessed via visual field defects and retinal nerve fiber layer changes) and retinal venule oxygen saturation. [30] The study also revealed negative correlations between damage and arteriovenous oxygen saturation differences. The finding of increased oxygen saturation being associated with severity of visual field damage was re-demonstrated in a recent study using the Zilia system.[31] Additional research has demonstrated mild oxygenation changes following glaucoma treatment, correlating with intraocular pressure modifications.[32]
Inherited Retinal Diseases
Studies of retinal oximetry in both adult and pediatric populations with inherited retinal diseases have shown reproducible and significant differences in oxygen consumption. Oxygen saturation increases in patients with inherited retinal diseases, particularly rod-cone dystrophy, due to decreased oxygen consumption. This increased saturation may contribute to photoreceptor and retinal degeneration through oxygen free radical accumulation. These changes correlate with structural and functional alterations, suggesting potential applications as biomarkers for disease progression and treatment response monitoring. [33][34][35][36][37]
Emerging Applications
Retinal oximetry applications continue to expand beyond traditional vascular ophthalmic conditions. Recent research has shown the potential of retinal oximetry in uveitis,[38] age-related macular degeneration,[39] and as a measure of optical clarity in cataract,[40] as well as glaucoma and inherited retinal diseases as above. Furthermore, retinal oximetry may serve as a biomarker for systemic conditions. Early research suggests applications in cardiopulmonary disease assessment and systemic oxygenation monitoring.[41][42][43] Studies exploring relationships between retinal vascular changes and Alzheimer's disease in the central nervous system show particular promise.[44][45] As technology advances, both the methodology and applications of retinal oximetry continue to evolve.
Additional Resources
References
- ↑ Hayreh SS, Zimmerman MB, Kimura A, Sanon A. Central retinal artery occlusion.: Retinal survival time. Experimental eye research. 2004;78(3):723-36.
- ↑ Linsenmeier RA, Zhang HF. Retinal oxygen: from animals to humans. Progress in retinal and eye research. 2017;58:115-51.
- ↑ Linsenmeier RA, Zhang HF. Retinal oxygen: from animals to humans. Prog Retin Eye Res. 2017 May;58:115-151. doi: 10.1016/j.preteyeres.2017.01.003. Epub 2017 Jan 18. PMID: 28109737; PMCID: PMC5441959.
- ↑ Garg AK, Knight D, Lando L, Chao DL. Advances in retinal oximetry. Transl Vis Sci Technol 2021;105
- ↑ Patel SR, Flanagan JG, Shahidi AM, Sylvestre JP, Hudson C. A prototype hyperspectral system with a tunable laser source for retinal vessel imaging. Invest Ophthalmol Vis Sci. 2013;54(8):5163-8.
- ↑ Shu X, Beckmann L, Zhang HF. Visible-light optical coherence tomography: a review. Journal of biomedical optics. 2017;22(12):121707-.
- ↑ Song W, Wei Q, Liu W, Liu T, Yi J, Sheibani N, et al. A combined method to quantify the retinal metabolic rate of oxygen using photoacoustic ophthalmoscopy and optical coherence tomography. Scientific reports. 2014;4(1):6525.
- ↑ Jump up to: 8.0 8.1 Beach JM, Schwenzer KJ, Srinivas S, Kim D, Tiedeman JS. Oximetry of retinal vessels by dual-wavelength imaging: calibration and influence of pigmentation. J Appl Physiol. 1999;86:748–758.
- ↑ Jump up to: 9.0 9.1 Hardarson SH. Retinal oximetry. Acta Ophthalmologica. 2012;91: 489–490.
- ↑ Jump up to: 10.0 10.1 Hardarson SH, Harris A, Karlsson RA, et al. Automatic retinal oximetry. Invest Ophthalmol Vis Sci. 2006; 47(11):5011-5016.
- ↑ Zilia. About Us. Available from: https://ziliahealth.com/about/. Accessed February 24, 2025.
- ↑ Smith JD, Bisignano K, Harrison WW. Test-retest repeatability in macular retinal oximetry. Clinical and Experimental Optometry. 2024 Aug 17;107(6):616-21.
- ↑ Jani PD, Mwanza JC, Billow KB, et al. Normative values and predictors of retinal oxygen saturation. Retina. 34(2):394-401, 2014
- ↑ Hardarson SH, and E Stefánsson. "Oxygen saturation in central retinal vein occlusion." American journal of ophthalmology 150.6 (2010): 871-875.
- ↑ Jump up to: 15.0 15.1 15.2 Boeckaert J., E. Vandewalle, and Ingeborg Stalmans. "Oximetry: recent insights into retinal vasopathies and glaucoma." Bull Soc Belge Ophtalmol 319 (2012): 75-83.
- ↑ Yang JY, You B, Wang Q, Chan SY, Jonas JB, Wei WB. Retinal vessel oxygen saturation in healthy subjects and early branch retinal vein occlusion. Int J Ophthalmol. 2017;10(2):267-70.
- ↑ Hardarson SH, Stefánsson E. Oxygen saturation in branch retinal vein occlusion. Acta Ophthalmol. 2012;90(5):466-70.
- ↑ Šínová I, Řehák J, Nekolová J, Jirásková N, Haluzová P, Řeháková T, et al. Correlation Between Ischemic Index of Retinal Vein Occlusion and Oxygen Saturation in Retinal Vessels. Am J Ophthalmol. 2018;188:74-80.
- ↑ Hammer M, Vilser W, Riemer T, et al. Diabetic patients with retinopathy show increased retinal venous oxygen saturation. Graefes Arch Clin Exp Ophthalmol. 2009;247:1025-1030.
- ↑ Khoobehi B, Firn K, Thompson H, Reinoso M, Beach J. Retinal arterial and venous oxygen saturation is altered in diabetic patients. Invest Ophthalmol Vis Sci. 2013;54:7103–7106.
- ↑ Jump up to: 21.0 21.1 21.2 Stefansson E et al. Retinal oximetry: Metabolic imaging for diseases of the retina and brain, Progress in Retinal and Eye Research, Volume 70, 2019, Pages 1-22,
- ↑ Smith JD, Sapoznik KA, Bisignano K, Benoit J, Harrison WW. Evaluation of macular retinal oximetry across different levels of diabetic retinopathy: a cross sectional study. BMC ophthalmology. 2025 Jan 17;25(1):24.
- ↑ C.M. Jorgensen, S.H. Hardarson, T. Bek. The oxygen saturation in retinal vessels from diabetic patients depends on the severity and type of vision-threatening retinopathy. Acta Ophthalmol., 92 (2014), pp. 34-39
- ↑ B. Khoobehi, K. Firn, H. Thompson, M. Reinoso, J. Beach. Retinal arterial and venous oxygen saturation is altered in diabetic patient. Invest Ophthalmol Vis Sci, 54 (2013), pp. 7103-7106
- ↑ C. Jorgensen, T. Bek. Increasing oxygen saturation in larger retinal vessels after photocoagulation for diabetic retinopathy. Invest Ophthalmol Vis Sci, 55 (2014), pp. 5365-5369
- ↑ Blair NP, Wanek J, Felder AE, Joslin CE, Kresovich JK, Lim JI, et al. Retinal Oximetry and Vessel Diameter Measurements With a Commercially Available Scanning Laser Ophthalmoscope in Diabetic Retinopathy. Invest Ophthalmol Vis Sci. 2017;58(12):5556-63.
- ↑ Kashani AH, Lopez Jaime GR, Saati S, Martin G, Varma R, Humayun MS. Noninvasive assessment of retinal vascular oxygen content among normal and diabetic human subjects: a study using hyperspectral computed tomographic imaging spectroscopy. Retina. 2014;34(9):1854-60.
- ↑ Olafsdottir Olof Birna, et al. "Retinal oximetry in primary open-angle glaucoma."Investigative ophthalmology & visual science 52.9 (2011): 6409-6413.
- ↑ Osborne N. N., et al. "Neuroprotection in relation to retinal ischemia and relevance to glaucoma." Survey of ophthalmology 43 (1999): S102-S128.
- ↑ Vandewalle E, Abegão Pinto L, Olafsdottir OB, De Clerck E, Stalmans P, Van Calster J, et al. Oximetry in glaucoma: correlation of metabolic change with structural and functional damage. Acta Ophthalmol. 2014;92(2):105-10.
- ↑ Mahmoudinezhad G, Moghimi S, Micheletti E, Du KH, Adelpour M, Latif K, Walker E, Salcedo M, Rubio V, Weinreb RN. Relationship Between Retinal Oxygen Saturation and the Severity of Visual Field Damage in Glaucoma. Journal of Glaucoma. 2024 Oct 1;33(10):728-34.
- ↑ S. Du, X. Gao, X. Zhang, J. Wang, W. Huang, M. Zhou, W. Wang, X. Li, Y. Zhang, D.S. LamChanges in retinal oxygen saturation, choroidal thickness. , and retinal nerve fibre layer. Can. J. Ophthalmol., 50 (2015), pp. 159-165
- ↑ Bojinova, R.I.; Türksever, C.; Schötzau, A.; Valmaggia, C.; Schorderet, D.F.; Todorova, M.G. Reduced metabolic function and structural alterations in inherited retinal dystrophies: Investigating the effect of peripapillary vessel oxygen saturation and vascular diameter on the retinal nerve fibre layer thickness. Acta Ophthalmol. 2017, 95, 252–261.
- ↑ Della-Volpe, W.M.; Scholl, H.P.N.; Valmaggia, C.; Todorova, M.G. Retinal vessel oximetry in children with inherited retinal diseases. Acta Ophthalmol. 2020
- ↑ Türksever C, López Torres LT, Valmaggia C, Todorova MG. Retinal Oxygenation in Inherited Diseases of the Retina. Genes (Basel). 2021 Feb 14;12(2):272. doi: 10.3390/genes12020272. PMID: 33672973; PMCID: PMC7918478.
- ↑ Parameswarappa DC, Kulkarni A, Sahoo NK, Padhy SK, Singh SR, Héon E, Chhablani J. From Cellular to Metabolic: Advances in Imaging of Inherited Retinal Diseases. Diagnostics. 2024 Dec 26;15(1):28.
- ↑ Prétot D, della Volpe Waizel M, Kaminska K, Valmaggia P, Placidi G, Falsini B, Fries FN, Szentmáry N, Rivolta C, Scholl HP, Calzetti G. Retinal oxygen metabolic function in choroideremia and retinitis pigmentosa. Graefe's Archive for Clinical and Experimental Ophthalmology. 2024 Oct 12:1-7.
- ↑ Abu El‐Asrar AM, AlBloushi AF, Alzubaidi A, Gikandi PW, Stefánsson E. Pretreatment ocular blood flow and retinal oxygen metabolism in the acute uveitic phase is associated with final outcome in Vogt‐Koyanagi‐Harada disease. Acta Ophthalmologica. 2024 Sep;102(6):720-7.
- ↑ Geirsdottir A, Hardarson SH, Olafsdottir OB, Stefánsson E. Retinal oxygen metabolism in exudative age‐related macular degeneration. Acta ophthalmologica. 2014 Feb;92(1):27-33.
- ↑ Klefter ON, Erichsen JH, Hansen MM, Holm LM, Hardarson SH, Stefánsson E, Kessel L. Evaluation of a retinal oximetry image quality indicator in patients with cataract. Acta Ophthalmologica. 2024 May;102(3):312-7.
- ↑ Hoel S, Moe K, Sugulle M, Petrovski G, Veiby NC, Staff AC. Retinal oximetry and microvascular assessment after hypertensive pregnancy complications. Acta Ophthalmologica. 2024 Sep;102(6):653-61.
- ↑ Stefánsson E, Olafsdottir OB, Eliasdottir TS, Vehmeijer W, Einarsdottir AB, Bek T, Torp TL, Grauslund J, Eysteinsson T, Karlsson RA, Van Keer K. Retinal oximetry: metabolic imaging for diseases of the retina and brain. Progress in retinal and eye research. 2019 May 1;70:1-22.
- ↑ Eliasdottir TS. Retinal oximetry and systemic arterial oxygen levels. Acta Ophthalmologica. 2018 Nov;96:1-44.
- ↑ Einarsdottir AB, Hardarson SH, Kristjansdottir JV, Bragason DT, Snaedal J, Stefánsson E. Retinal oximetry imaging in Alzheimer’s disease. Journal of Alzheimer's Disease. 2016 Jan 1;49(1):79-83.
- ↑ Stefánsson E, Olafsdottir OB, Einarsdottir AB, Eliasdottir TS, Eysteinsson T, Vehmeijer W, Vandewalle E, Bek T, Hardarson SH. Retinal oximetry discovers novel biomarkers in retinal and brain diseases. Investigative ophthalmology & visual science. 2017 May 1;58(6):BIO227-33.