Retinal Capillary Hemangioblastoma and von Hippel-Lindau Disease
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
Introduction
Retinal capillary hemangioblastomas (RCH) (also known as retinal angiomas) can be a a sign of a von Hippel-Lindau (VHL) disease although they may also be observed as an isolated entity without systemic involvement. RCH is the most frequent and earliest manifestation of the VHL disease and therefore an ophthalmologist is often involved in the care of these patients. This article will touch open the important concepts of the retinal capillary hemangioblastoma, a cardinal feature of VHL syndrome. Further information about the other systemic manifestations of VHL syndrome can be found in the resources section of the article.
International classification of diseases (ICD)
Von-Hippel Lindau Disease
von Hippel-Lindau disease is a heritable multisystem syndrome that is associated with a germline mutation of the VHL tumour suppressor gene on the short arm of chromosome 3. The incidence of this disorder is approximately 1 in 36,000 live births and it is inherited in a high penetrance autosomal dominant pattern. This disease is characterized by the growth of various benign or malignant tumors of the retina and the brain, along with cysts of several visceral organs such as the kidneys, pancreas, and adrenal glands and reproductive organs.[1] Therefore, the management of such patients necessitates a multi-disciplinary approach.
History
The name of VHL disease derives from two prestigious European physicians, Eugen von Hippel and Arvid Lindau; however, others
have contributed as well in the recognition of the syndrome. Early observers of the syndrome were the English neurologist John Hughlings Jackson (1872) and the German ophthalmologist Hugo Magnus (1874).[2] Ernst Fuchs described this angiomatous condition in the retina in 1882 followed by Treacher Collins (1894) who noticed its inherited nature[3][4]. A few years later, in 1904, Eugen von Hippel (August 3, 1867 - September 5, 1939) a German ophthalmologist, who studied medicine in Heidelberg under Theodore Leber described the retinal angiomas, in his seminal paper entitled “Ueber eine sehr seltene Erkrankung der Netzhaut ( transl : About a very rare disease of the retina”), which he named "angiomatosis retinae"[5]. Coats described a similar condition as well in 1908.[6]
The typical association of angiomas of the retina with the cerebellum was first described in 1905 by the Prague ophthalmologist Wilhelm Czermak, long before Arvid Lindau (1926).[7] Lindau, a Swedish pathologist, wrote a thesis about the association between cerebellar and retinal capillary hemangiomas as a heritable entity. He described his observations in his thesis entitled "Studien über Kleinhirncysten. Bau, Pathogenese und Beziehungen zur Angiomatosae retinae (transl : Studies about cerebellar
cysts. Structure, pathogenesis and relationship with angiomatosis retinae).[8]
Lindau’s work draw the attention of the neurosurgeon Harvey Cushing, who published a case of cerebellar hemangioma referring to this entity as “Lindau’s disease”.[9] The term von Hippel-Lindau disease was first used in 1936 by Davison [10], but it was not used commonly until the 1970s.
Pathophysiology
VHL results from a germline mutation in the VHL gene which is a tumour suppressor gene located on the short arm of chromosome 3 (3p25–26). This discovery by Latif and colleagues in 1993 was a seminal step in the understanding of the molecular pathology of VHL disease.[11] It is now believed that tumor formation in VHL disease follows the "2-hit model," initially hypothesized by Knudson for retinoblastoma i.e affected individuals inherit a mutated VHL gene which is present in all the cells of affected individuals.[12] However, only those cells that undergo a deletion or mutation of the remaining wild type allele, and are constituents of susceptible target organ will undergo tumor formation.
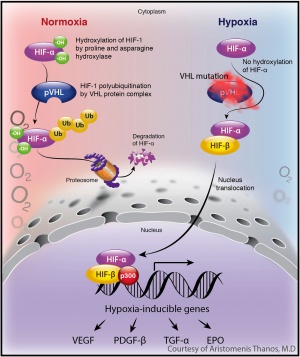
VHL gene encodes the VHL protein, which is a tumor suppressor protein. The protein forms a complex with other proteins including elongin B, elongin C, and Cullin 2 (CUL2), to form the
VCB- CUL2 complex. This protein complex plays an important role in the ubiquitin-mediated degradation of intracellular proteins through the proteasome. Under normoxic conditions, the VHL protein complex binds to, and targets, the alpha subunits of hypoxia-inducible factors (HIF) 1 and 2 for ubiquitin-mediated degradation by the proteasome. HIF1a and HIF2 are potent transcriptional factors that are important for the cellular response under hypoxic conditions, since they enhance glucose uptake and increase expression of angiogenic, growth, and mitogenic factors including vascular endothelial growth factor (VEGF), platelet derived growth factor (PDGF), erythropoietin (EPO) , and transforming growth factor (TGF).[13]
Clinical Presentation
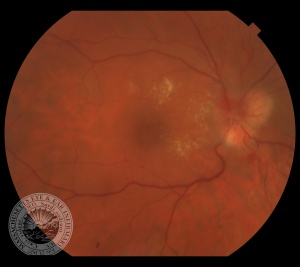
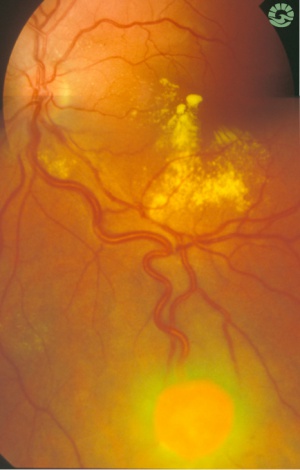
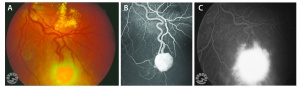
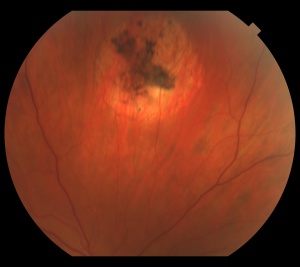
The retinal capillary hemangioblastoma (RCH) is the most frequent and earliest manifestation of VHL disease with a mean age of diagnosis of 25 years.[14][15][16] The clinical presentation of retinal capillary hemangioblastoma varies greatly depending on the size and location of the tumor. In a large cross sectional study performed by the National Eye Institute where 335 VHL patients were enrolled, RCH was was unilateral in 42% of cases and bilateral in 58%. Peripheral lesions were identified in approximately 85% of patients and juxtapapillary lesions in 15%.[17] Peripheral lesions have often a subtle red hue and they are no larger than a few hundred microns. As the proliferation continues, they develop a more nodular appearance leading to a characteristic clinical appearance with marked dilated and engorged afferent and efferent blood vessels. Retinal edema and hard exudates are often seen in association with the tumor and they may often involve the macula. Juxtapapillary lesions, encountered in approximately 11-15% of cases, can cause pseudopapilledema from elevation and exudation around the optic nerve and it can be the sole manifestation of retinal VHL disease.[18] Retinal or vitreous hemorrhages are rarely observed, occurring in less than 3% of cases.[19]
Clinical Diagnosis
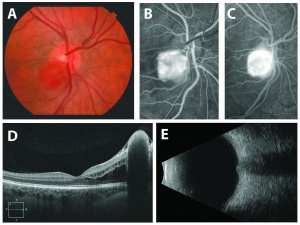
The diagnosis of the retinal capillary hemangioblastoma is primarily clinical. Dilated fundus exam is essential in identifying all the existing RCHs, as they may reach up to 11 in number.[20] A classic diagnostic finding is the dilated, tortuous vessels leading to and away of the vascular tumor. Fundus photography, especially ultra-widefield retinal imaging to capture the location, number, and size of the peripheral lesions may be helpful in following the growth or regression of the lesions. Fluorescein angiography typically shows early leakage and marked hyperfluorescence. Macular edema associated with these lesions may also be detected by means of optical coherence tomography.
Differential Diagnosis
The diagnosis of retinal capillary hemangioblastoma (RCH) can be made most of times based on dilated fundus examination together with careful questioning of the past medical (if any) and/or family history. Below, is a list with the possible conditions that can mimic a RCH :
- Coat's disease
- Racemose hemangioma
- Retinal cavernous hemangioma
- Retinal macroaneurysm
- Vasoproliferative tumor
In addition, juxtapapillary RCH can mimic papilledema, papillitis or choroidal neovascular membrane.
Causes of vision loss
In the largest cohort study performed by the NEI, risk factors for associated vision loss were age of the patient and location of the tumor.[21] The risk of severe vision loss also increased with the presence of juxtapapillary lesions and increasing peripheral tumor number and the extent of the retinal involvement. In the same study, approximately 77% of patients had vision of 20/20 or better whereas the overall prevalence of legal blindness from ocular VHL was low at 5.7% with vision less than 20/160 in the better-seeing eye. In summary, vision loss from RCH can occur through a variety of mechanisms listed below :
- Exudation : increase in capillary tumor vasopermeablity leading to macular edema or exudative retinal detachment.
- Tractional effects : glial proliferation on the surface of the tumor may induce retinal striae & distortion or even tractional retinal detachment
- Vitreous hemorrhage : from rupture and bleeding of the RCH into the vitreous cavity
- Neovascular glaucoma : leaking of angiogenic factors, such as VEGF, to the anterior chamber causing neovascularization of the angle
Histopathology
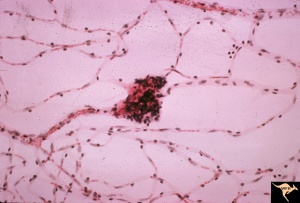
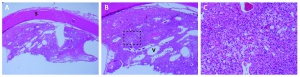
The three major types of cells of the retinal capillary hemangioblastoma are endothelial cells, pericytes, and "foamy" stromal cells.[22] The endothelial cells are fenestrated, providing the basis for the exudation that is characteristic of the tumor. [23]The stromal cells contain abundant lipid vacuoles and a few organelles. In the past, ultrastructural and immunohistochemical studies have suggested that these “stromal” cells may represent lipidized fibrous astrocytes or glial cells.[24]
Using tissue microdissection and polymerase chain reaction amplification techniques, Chan and coworkers showed that the “stromal” cells of the retinal angioma have a complete loss of VHL gene and enhanced VEGF gene expression. [25]The pVHL, the VHL suppressor gene product, is known to downregulate vascular endothelial growth factor (VEGF) expression. With the absence of pVHL, VEGF is upregulated, resulting in neovascularization on and around these retinal capillary hemangioblastomas. This study provided a very strong evidence that the vacuolated stromal cells represent the true neoplastic component of retinal angiomas. This is further supported by animal studies, where VHL gene has been selectively ablated in astrocytes using Cre-LoxP conditional gene targeting techniques, recapitulating the phenotype of human disease. [26][27]
Treatment
Various treatment modalities have been reported in the literature for the management of RCH, including observation, laser photocoagulation, cryotherapy, plaque and proton beam radiotherapy, and vitreoretinal surgery. It is important to notice that the efficacy and applicability of these modalities are very much influenced by the
location of the tumor (peripheral vs juxtapapillary), size of the tumor and finally the presence of any associated findings (e.g subretinal fluid, exudation, evidence of traction).
Observation
Very small, non-visually-threatening peripheral lesions (< 500uM) with no associated exudation can be observed as they may undergo regression or remain stable. Observation is often preferred in cases of juxtapapillary hemangiomas, where the application of other treatment modalities is associated with significant morbidity.
Laser Photocoagulation
Argon laser photocoagulation has been the mainstay of treatment for peripheral lesions less than 3.00mm with no associated subretinal fluid. Several studies have studied the efficacy of Argon Laser Photocoagulation in the treatment of RCH and are summarized in the following table (only studies with > 30 RCH were included)
| First Author | Year | No. of RCHs included in study | Size(mm) | Laser method |
Control Rate |
|---|---|---|---|---|---|
| Schmidt[28] | 2000 | 100 | <3.00 | Argon | 91% |
| Gorin[29] | 1992 | 55 | <3.00 | Argon direct & feeder | 96% |
| Bonnet[30] | 1984 | 36 | N/A | Argon | 100% |
Cryotherapy
In cases where significant subretinal fluid is present, the RCH is located anteriorly and the tumor size larger is than 3.00mm, cryotherapy is the preferred modality. Lincoff was among the pioneers who described the use of cryotherapy for the management of RCH.[31] The cryotherapy technique is the one originally described by Welch. [32] Temperatures of -80oC and no more than 2 freeze cycles are applied to control the tumor.
Anti-VEGF
Use of anti-VEGF has been proposed as an adjunct in the management of RCHs. Unfortunately, there is no large clinical trial to date examining the efficacy of anti-VEGF agents in the management of VHL patients. In a small cohort study performed by the National Eye Institute, ranibizumab (Lucentis) treatment demonstrated promising, but minimal, success on most VHL-related hemangioblastomas. The study concluded that ranibuzimab may aid in control of associated findings (i.e decrease in subretinal fluid) but had limited efficacy on the RCH itself.[33]
Surgical Excision
Very rarely, RCH tumors can cause lead to a retinal detachment. If this is exudative, observation may remain the best management options, but if there is a tractional or rhegmatogenous component, then vitreoretinal surgery may be necessary. There have been a handful of reports in the literature describing techniques that have been utilized in excising a retinal capillary hemangioblastoma.[34][35][36][37] While clinical outcomes have been mixed, there have been several cases with reported visual improvement postoperatively. See this LINK for a surgical video demonstrating one technique of RCH resection.
Other Treatment Modalities
Recently, small studies have shown that belzutifan, an oral HIF-2 alpha inhibitor, has been effective in regressing these tumors[38]. Photodynamic dynamic therapy[39] and proton beam irradiation[40] have also been utilized with variable results, but more data is needed.[41]
Additional Resources
- http://en.wikipedia.org/wiki/Von_Hippel%E2%80%93Lindau_disease
- http://www.vhl.org/
- http://www.ninds.nih.gov/disorders/von_hippel_lindau/von_hippel_lindau.htm
- http://ghr.nlm.nih.gov/condition/von-hippel-lindau-syndrome
- http://omim.org/entry/193300
- https://eyewiki.org/Intraocular_Vascular_Tumors
References
- ↑ Maher, ER; Neumann, HP; Richard, S (2011 Jun). "von Hippel-Lindau disease: a clinical and scientific review.". European journal of human genetics : EJHG 19 (6): 617-23
- ↑ H Magnus. Aneurysma arteriovenosum retinale. Virch Arch Path Anat, 60 (1874), pp. 38–44
- ↑ Fuchs EF Aneurysma arterio-venosum retinae. Arch Augeneheild. 1882;11440- 444
- ↑ Collins ET. Intra-ocular growths (two cases, brother and sister, with peculiar vascular new growth, probably primarily retinal, affecting both eyes). Trans Opthalmol Soc U K. 1894;14 : 141- 149
- ↑ von Hippel E Uber eine sehr seltene Erkrankung der Netzhaut. Klin Beobachtungen Arch Ophthalmol. 1904;59:83- 106
- ↑ Coats G Forms of retinal disease with massive exudation. R Lond Ophthal Hosp Rep. 1908;17440- 496
- ↑ W Czermak. Pathologisch-anatomischer Befund bei der Von E. v. Hippel beschriebenen sehr seltenen Netzhauterkrankung. Ber Dtsch Ophthal Ges, 32 (1906), pp. 184–195
- ↑ Lindau A Zur Frage der Angiomatosis retinae und ihrer Hirnkomplikationen. Acta Ophthalmol. 1927;4193- 226
- ↑ Cushing H, Bailey P. Hemangiomas of the cerebellum and retina (lindau’s disease) ; with the report of a case . Arch Ophthalmol. 1928;57 : 447-63
- ↑ Davison C, Brock S, Dyke CG. Retinal and centralfckLRnervous hemangioblastomatosis with visceral changes (von Hippel-Lindau’s disease). Bull Neurol Instit NY 1936;5:72–93.
- ↑ Latif, F. et al. Identification of the von Hippel-Lindau disease tumour suppressor gene. Science 260, 1317−1320 (1993)
- ↑ Knudson, A.G. Hereditary cancer, oncogenes, and antloncogenes. Cancer Res. 45, 1437−1443.
- ↑ Maina H et al. Identification of novel VHL target genes and relationship to hypoxic response pathways. Oncogene (2005) 24, 4549–4558.
- ↑ W.A Horton, V Wong, R Eldridge : von Hippel–Lindau diseaseclinical and pathological manifestations in nine families with 50 affected members. Arch Intern Med, 136 (1976), pp. 769–777
- ↑ E.R Maher, J.R Yates, R Harries et al.Clinical features and natural history of von Hippel–Lindau disease. QJM, 77 (1990), pp. 1151–1163
- ↑ J.M Lamiell, F.G Salazar, Y.E Hsia. von Hippel–Lindau disease affecting 43 members of a single kindred. Medicine (Baltimore), 68 (1989), pp. 1–29
- ↑ Wong WT, Agrón E, Coleman HR, et al: Clinical characterization of retinal capillary hemangioblastomas in a large population of patients with von Hippel–Lindau disease. Ophthalmology 2008; 115:181-188.
- ↑ J.A Oosterhuis, K Rubinstein.Haemangioma at the optic disc. Ophthalmologica, 164 (1972), pp. 362–374
- ↑ A.R Webster, E.R Maher, A.T MoorefckLRClinical characteristics of ocular angiomatosis in von Hippel–Lindau disease and correlation with germline mutation. Arch Ophthalmol, 117 (1999), pp. 371–378
- ↑ Wong WT, Agrón E, Coleman HR, et al: Clinical characterization of retinal capillary hemangioblastomas in a large population of patients with von Hippel–Lindau disease. Ophthalmology 2008; 115:181-188.
- ↑ Wong WT, Agrón E, Coleman HR, et al: Clinical characterization of retinal capillary hemangioblastomas in a large population of patients with von Hippel–Lindau disease. Ophthalmology 2008; 115:181-188.
- ↑ Green WR: Retina: capillary hemangioma. In: Spencer WH, ed. Ophthalmic pathology: An atlas and textbook, Philadelphia: WB Saunders; 1996:709-718.
- ↑ Jakobiec FA, Font RL, Johnson FB: Angiomatosis retinae: an ultrastructural study and lipid analyses. Cancer 1976; 38:2042-2056.
- ↑ Jakobiec FA, Font RL, Johnson FB: Angiomatosis retinae: an ultrastructural study and lipid analyses. Cancer 1976; 38:2042-2056.
- ↑ Chan CC, Vortmeyer AO, Chew , et al: VHL gene deletion and enhanced VEGF gene expression detected in the stromal cells of retinal angioma. Arch Ophthalmol 1999; 117:625-630
- ↑ Kurihara T., Kubota Y., Ozawa Y., Takubo K., Noda K., Simon M.C., Johnson R.S., Suematsu M., Tsubota K., Ishida S., et al. 2010. von Hippel-Lindau protein regulates transition from the fetal to the adult circulatory system in retina. Development. 137:1563–1571. doi: 10.1242/dev.049015.
- ↑ Kurihara T, Westenskow PD, Krohne TU, Aguilar E, Johnson RS, Friedlander M. Astrocyte pVHL and HIF-alpha isoforms are required for embryonic-to-adult vascular transition in the eye. J Cell Biol. 2011;195(4):689–701
- ↑ D Schmidt, E Natt, H.P Neumann.Long-term results of laser treatment for retinal angiomatosis in von Hippel–Lindau disease.Eur J Med Res, 5 (2000), pp. 47–58
- ↑ M.B Gorin.von Hippel–Lindau disease clinical considerations and the use of fluorescein-potentiated argon laser therapy for treatment of retinal angiomas. Semin Ophthalmol, 7 (1992), pp. 182–191
- ↑ Bonnet M, Garmier G, Tlouzeau S, Burtin C: [Treatment of retinal capillary angiomas of von Hippels disease]. J Fr Ophtalmol 7:545–55, 1984
- ↑ H Lincoff, J McLean, R Long The cryosurgical treatment of intraocular tumors Am J Ophthalmol, 63 (1967), pp. 389–399
- ↑ R.B Welch von Hippel–Lindau diseasethe recognition and treatment of early angiomatosis retinae and the use of cryosurgery as an adjunct to therapy Trans Am Ophthalmol Soc, 68 (1970), pp. 367–424
- ↑ Wong WT, Liang KJ, Hammel K, et al. Intravitreal ranibizumab therapy for retinal capillary hemangioblastoma related to von Hippel-Landau disease. Ophthalmology. 2008;115(11 ):1957–64
- ↑ Schlesinger T, Appukuttan B, Hwang T, et al. Internal en bloc resection and genetic analysis of retinal capillary hemangioblastoma. Arch Ophthalmol. 2007 Sep;125(9):1189-93.
- ↑ Khurshid GS.Transvitreal endoresection of refractory retinal capillary hemangioblastoma after feeder vessel ligation. Ophthalmic Surg Lasers Imaging Retina. 2013 May-Jun;44(3):278-80.
- ↑ Weng CY, Shetlar DJ, Beltran B. En Bloc Resection of a Retinal Capillary Hemangioblastoma in a Young Female. Ophthalmology. 2018 Aug;125(8):1188.
- ↑ Weng CY. Transvitreal Feeder Vessel Ligation and En Bloc Resection of a Retinal Capillary Hemangioblastoma. Am J Ophthalmol. 2022 May;237:e3-e5. doi: 10.1016/j.ajo.2022.01.007. Epub 2022 Jan 14. PMID: 35033541.
- ↑ Wiley HE, Srinivasan R, Maranchie JK, Chhablani J, Bøndergaard Iversen AB, Kruse A, Jonasch E, Gombos DS, Else T, Demirci H, Maughan BL, Hartnett ME, Coleman HR, Fu W, Perini RF, Liu Y, Linehan WM, Chew EY; LITESPARK-004 Investigator Group – Ocular VHL. Oral HIF-2α Inhibitor Belzutifan in Ocular von Hippel-Lindau Disease: Subgroup Analysis of the Single-Arm Phase 2 LITESPARK-004 Study. Ophthalmology. 2024 Jun 5:S0161-6420(24)00319-1. doi: 10.1016/j.ophtha.2024.05.024. Epub ahead of print. PMID: 38849055.
- ↑ Barbazetto IA, Schmidt-Erfurth UM: Photodynamic therapy in the treatment of intraocular angioma[abstract]. Presented at the American Academy of Ophthalmology. Orlando, FL, USA, 1999
- ↑ J.D Palmer, E.S Gragoudas Advances in treatment of retinal angiomas Int Ophthalmol Clin, 37 (1997), pp. 150–170
- ↑ J.D Palmer, E.S Gragoudas. Advances in treatment of retinal angiomas. Int Ophthalmol Clin, 37 (1997), pp. 150–170