Refractive Error After Cataract Surgery
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
Disease Entity
Cataract surgery outcomes have greatly improved due to advancements in surgical technique, intraocular lens (IOL) technology, and preoperative testing and calculations. With the improvements have come increased expectations from patients regarding postoperative visual acuity and independence from spectacle correction. Studies on cataract surgery outcomes show that 50-70% and 79-94% of patients will achieve postoperative refractions within 0.5 D and 1.0 D of the intended target, respectively.[1][2][3][4] Toric IOLs as well as limbal relaxing incisions and astigmatic keratectomy now provide the opportunity to correct astigmatism with good results. A study of patients undergoing placement of toric IOLs found that 88% had less than 1.0 D of astigmatism postoperatively.[5] If refractive error does occur after surgery, there are numerous options that may provide the patient with a satisfactory final outcome. These are especially important in certain populations such as patients with a history of keratorefractive surgery where there are higher rates of postoperative refractive error or patients undergoing premium IOL placement who are more sensitive to refractive error. Refractive error after cataract surgery may be decreasing but is still a relatively common occurrence that impacts patient satisfaction. Therefore, cataract surgeons should take all precautions to prevent its occurrence as well as diagnose and manage the refractive error effectively.
Disease
Refractive error after cataract surgery typically manifests with blurred vision at distances where the patient was expecting to have good uncorrected visual acuity. Patients who are 20/20 uncorrected at distance with plano refraction may be unhappy if the goal was clear near vision. The amount of deviation from the target refraction at which the patient becomes symptomatic is largely dependent on the individual. The most commonly used end-points for measuring refractive error in the literature are the percentage of patients achieving final refraction within 0.5 D and 1.0 D of the intended target.[6] These intervals are the highest practical levels of accuracy, as IOL powers change in 0.5 D increments. The expectation of spectacle independence at distance, near, or both in the cases of premium IOLs has led to dissatisfaction with cataract surgery that does not result in spectacle independence. Thus, even though refractive error may be corrected with glasses or contact lenses, patients are often not happy with this result. Another issue that refractive error may create is anisometropia if the refractive error is unilateral or asymmetric. This is usually quite symptomatic and requires additional surgery.
The importance of refractive predictability has become increasingly important since the advent of premium IOLs. Bifocal, trifocal, extended depth of focus (EDOF), and pseudo-accommodative lenses require precision in the post-operative refraction to maximize visual acuity. These lenses are associated with an increased rate of visual phenomena such as glare, halos, and night vision problems that are significantly worsened by any refractive error. Contrast sensitivity and subjective visual acuity are also disproportionately affected in these patients if any refractive error is present.[7]
Etiology
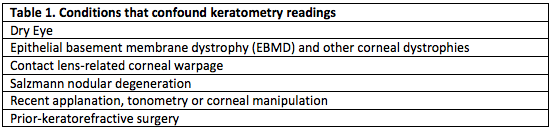
Accurate preoperative measurements are necessary to achieve adequate refractive outcomes in cataract surgery. Table 1 lists pre-existing conditions that may affect keratometry readings and Table 2 lists conditions that confound axial length measurements.[8] Treatment of dry eye disease is imperative for obtaining more accurate biometry calculations.
Patients with a history of refractive surgery have a higher likelihood of refractive error after cataract surgery. Reasons for this and a more extensive discussion may be found at Intraocular lens power calculation after corneal refractive surgery. Patients often will not know whether the surgery was hyperopic or myopic refractive surgery. Myopic ablations are typically done in patients in their 20s to 30s, eyes with longer axial length, and result in flatter central corneas (oblate). Hyperopic ablations are more commonly performed in older patients, eyes with shorter axial length, and result in steeper than normal corneal curvature.[8] In cases of PRK, it can be impossible to determine if there was prior surgery on exam, and despite thorough history taking, patients may not volunteer this information. Hyperopic surprise most commonly results if the history of refractive surgery is not taken into account when calculating IOL power. Topography can be helpful in determining whether myopic or hyperopic excimer laser ablation has occurred.
All patients should be asked about contact lens use, and if present, the specific type and date of last use must be noted. Patients must stop soft contact lens use one week and rigid gas permeable contact lenses (RGPs) at least one month prior to pre-operative testing. After 1 month out of RGPs, topography should be done and then may be considered to be repeated 2-4 weeks later. Keratometry values may only be used if stable, as time to stability varies widely based on the individual. Over half of soft contact lens wearers show no change in topography after stopping contact lens use, whereas one review found that 44% of patients with long-term history of RGP use required longer than 6 weeks to achieve refractive stability.[9][10]
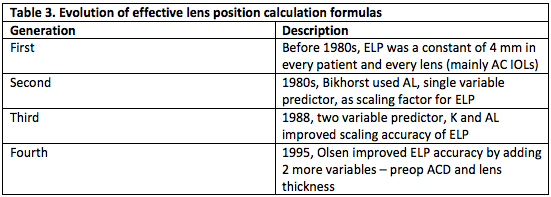
Eyes with axial lengths shorter or longer than the normal range classically had a high rate of refractive error after cataract surgery. This issue has improved due, in part, to the implementation of newer generation IOL calculation formulas, which are outlined in the Table 3. An excellent discussion of IOL calculation formulas and the differences between them may be found on Biometry for IOL calculations. Olsen and Barrett Universal II perform better for a wide range of axial lengths[11], even outperforming the third generation SRK/T formula and the fourth generation Haigis formula in eyes with high axial myopia (>28 mm).[12] [13] One review found that the newer Hill-RBF version 2.0 was comparable to Barrett Universal II and Haigis in eyes with axial lengths > 26 mm[14]. It is also suggested to aim for low myopia in eyes with extreme axial length due to the frequency of hyperopic error and because these patients are accustomed to myopia.[15]
Keratoconic eyes have deeper anterior chamber depths and longer axial lengths, which may lead to hyperopic surprise due to error in the estimated lens position. One review found that the SRK II formula had the best refractive outcomes in mild keratoconus, whereas no formula performs particularly well in severe keratoconus.[16] Another more recent review found that the Barrett Universal II was best for milder forms of keratoconus, and the use of Pentacam keratometry instead of corneal power measured by optical biometers may minimize the hyperopic error[17]. Some authors advocate for the use of the standard keratometry value of 43.25 D and a target refraction of -2.0 D in severe keratoconus.[8] Aiming for more myopia, especially in more severe forms of keratoconus is advocated, in anticipation of hyperopic error. Toric IOLs should be avoided in all RGP and scleral contact lens wearers.[8]
Sources of error unrelated to the eye may also contribute to refractive error. These include poor patient cooperation, data entry error, and placement of the wrong lens into the wrong eye. The largest study on wrong IOL implantations was a retrospective review of all reported cases in Wales from 2003 to 2010. Of the 164 reported incidents, the following etiologies were most common:[18]
- Inaccurate biometry
- Wrong IOL selection
- Transcription errors
- Handwriting misinterpretations
Standardized measures must be put in place to recognize and avoid these errors with special attention to these causes.
Optimized lens constants for IOL biometry for many IOLs may be found online, which are based on empirical data collected from many surgeons. This site lists many optimized lens constants http://ocusoft.de/ulib/. These show reasonable accuracy but should only be viewed as starting points until a surgeon has sufficient data to calculate individualized lens constants. These are created based on the surgeon’s personal outcomes and are more accurate. Surgeons may create an optimized lens constant on the IOL master on this site: https://www.doctor-hill.com/iol-master/optimization.htm. Optimized lens constants for Lenstar may be created here https://www.doctor-hill.com/physicians/download.htm.
Primary Prevention
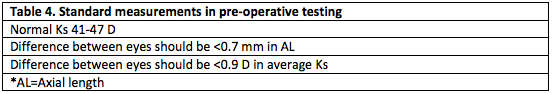
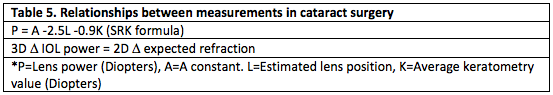
The ideal management of refractive error after cataract surgery is to prevent it from occurring. Prevention begins with thorough preoperative evaluation. History of any ocular surgery or contact lens use must be documented. Accurate preoperative measurements should be verified and repeated if they do not make sense. Surgeons should develop a systematic method for reviewing lens calculation sheets. Table 4 outlines when preoperative measurements should be questioned and Table 5 demonstrates how variables in IOL calculation are related.[19] Carefully examine for corneal irregularity, especially in contact lens patients. Evaluate the contralateral eye, and if significant differences exist between eyes, ensure that this matches preoperative refractive error.
Patients with a history of corneal refractive surgery represent a difficult subset of patients. This is due to an inability to accurately measure keratometric power in these eyes with currently available instrumentation. Pre-refractive surgery measurements, if available, may be helpful. A history of keratorefractive surgery will result in hyperopic surprise after cataract surgery due to overestimation of the corneal power if conventional formulas are utilized. For a more extensive discussion regarding cataract surgery in post-keratorefractive surgery patients, please see Intraocular lens power calculation after corneal refractive surgery.
Any measurement outside the normal range for axial length or keratometry should raise suspicion and further evaluation. There are higher rates of refractive error in eyes with long and short axial lengths. In cases of extreme axial myopia or hyperopia, conventional IOL prediction formulas will result in hyperopic surprise. Special preoperative planning and counseling, as outlined above, is recommended in these patients.
Diagnosis
It is critical to determine the etiology of refractive error after cataract surgery. This begins with an accurate refraction that is stable. The time to refractive stability may vary from 1 day to 3 months postoperatively. Patients with one-piece acrylic lenses may typically be refracted within the first week postoperatively whereas those with premium IOLs should not be refracted until postoperative month 1. Post-RK patients may take 3 months for refractive stability.[20] Auto-refraction is not adequate and subjective refraction is necessary.
A thorough dilated exam is critical to assess for corneal irregularity, lens malposition or distention, and/or retinal pathology. Corneal decompensation or irregularity due to surgery, eye drops, or trauma may result in pathology that was not present preoperatively. Preoperative measurements should be repeated and formulas used for surgery should be double-checked. An OCT of the macula may reveal clinically inapparent macular edema or other previously undetected pathology. If these measures do not identify a source of error, it may be assumed that there was an error in the estimated lens position and IOL formula used. A step-wise algorithm for determining the cause of refractive error after cataract surgery can be found in Table 6.
Management
Medical therapy
The first step in management of refractive error after cataract surgery are determining patient expectations. Patients who are asymptomatic and satisfied with visual outcomes after surgery may simply be observed. If patients are amenable to wearing glasses, spectacle correction should be the first option. Contact lenses may be preferable in cases of anisometropia, high astigmatism, or in patients accustomed to wearing contact lenses. It is also critical to have a frank discussion with the patient regarding the source of the refractive error, once it is known, and why this error occurred.
Surgery
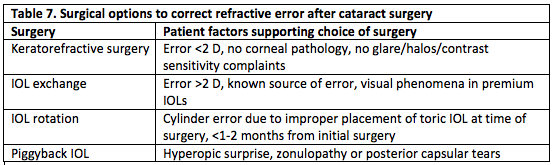
In patients with postoperative refractive error who desire spectacle independence, an extensive discussion regarding additional risks of further surgery is necessary. Any additional surgery carries the risk of loss of best corrected visual acuity, infection, and complications related to general anesthesia. These risks are often greater than in the initial cataract surgery. There are also significant costs to the patient, both financially and in time spent. Table 7 outlines surgical options and when they may be considered.
If the decision is made to proceed, one option is corneal refractive surgery. This is a highly accurate means of correcting residual refractive error with 92% of pseudophakes achieving a result within 0.5 D of the intended target. Additionally, it is very effective in MFIOL patients, as one large review showed that 90% of patients who underwent LASIK or PRK after MFIOL placement were within 0.5 D and 99.5% were within 1.0 D of the intended refractive target.[21] These patients all underwent conventional ablation and 99.2% of the patients were 20/40 or better at the final visit, demonstrating the safety of this option.[21]
It is recommended that LASIK be delayed 3 months after cataract surgery to allow for refractive and incisional stability. PRK may be pursued once manifest refraction is stable.[20] Thorough history and exam are necessary prior to LASIK to ensure that contraindications such as Fuchs’ dystrophy, epithelial basement membrane dystrophy (EBMD), severe dry eyes, or history of herpetic eye disease are not present. PRK may be used in many cases when LASIK is contraindicated due to corneal pathology.
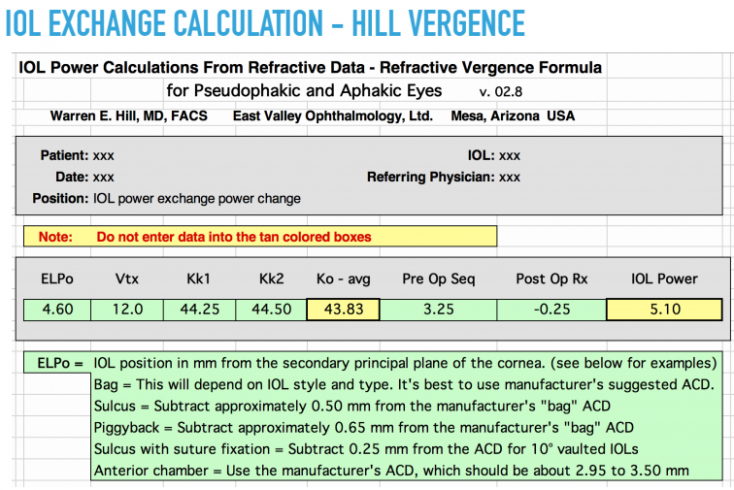
IOL exchange may be an effective option if the source of the error and the reason that it occurred are apparent. The IOL should be known and the same IOL platform should be used for the second surgery. Exchange is technically easiest to perform in the early post-operative period (within 4 months). Exchange is more commonly used in refractive errors greater than 1 D, as other methods such as corneal refractive surgery are more precise in correcting smaller degrees of refractive error. A vergence formula should be utilized for IOL exchange calculations, which can be found via this link: https://www.doctor-hill.com/physicians/download.html. A sample from this website is displayed in figure 1.
IOL exchange in premium IOL patients is a unique situation. These patients experience a higher rate of visual phenomena such as glare, halos, and decreased contrast sensitivity. It is recommended that surgeons wait longer to re-operate on these patients as these symptoms may diminish over months to years due to neuro-adaptation or gradual changes in lens position.[20] If symptoms persist, it is advisable to exchange the premium IOL for a monofocal IOL. Management options other than IOL exchange are unlikely to achieve satisfactory results in patients bothered by quality of vision or visual phenomena.[22]
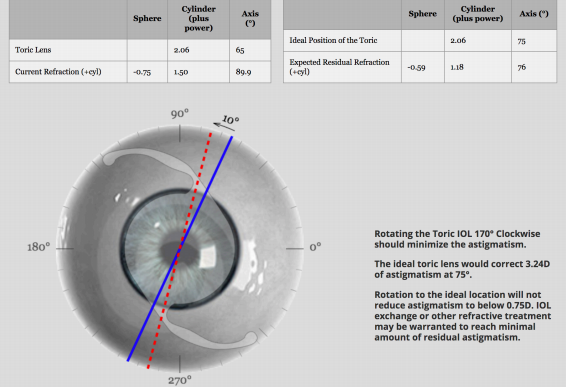
IOL rotation or repositioning may be the best option for patients with residual cylinder error after toric IOL placement. Rotation is indicated in cases where the IOL was placed in an improper position during surgery, not in cases where the IOL was initially properly placed but rotated in the postoperative period. IOL rotation should be done in the early postoperative period prior to the healing and fibrosis that occur within a few months of surgery, which make it difficult to change the lens orientation.[20] The postoperative axis of astigmatism should be used, rather than the values used at the time of the initial surgery. If it is not possible to reduce astigmatism to less than 0.75 D via IOL rotation, another option such as IOL exchange or keratorefractive surgery will likely be necessary. Specific IOL calculations for toric IOL exchange or rotation may be found at this website: https://www.astigmatismfix.com/. A sample from this website is shown in figure 2.
A piggyback IOL may be the optimal choice for patients with a hyperopic outcome, especially if the IOL power is not known. It is also an alternative to IOL exchange when the procedure would be high risk, such as in cases of posterior capsule tears or zonulopathy. For this procedure to be successful, the primary IOL must be fully in the capsular bag and the anterior chamber should be deep with an open angle to allow for adequate space for the secondary IOL. Silicone IOLs such as LI61 AO and Tecnis Z9002 are preferred, especially if the primary IOL was acrylic.[23] Square edge and acrylic IOLs should not be used for piggyback IOLs. This technique is associated with an increased risk of mechanical complications such as uveitis-glaucoma-hyphema (UGH) syndrome, iris chafing, and uveitis.
The following rules have been used in calculating IOL power for piggyback IOLs.
- Hyperopic error: 1.5 x manifest SE diopters
- Myopic error: 1.3 x manifest SE diopters
However, current vergence formulas (https://www.doctor-hill.com/physicians/download.html) provide more accurate IOL power calculations and should be utilized. It is always necessary to use these formulas in cases of refractive error greater than 7 D, as the above general rules become increasingly inaccurate with larger refractive error.[23]
As technology continues to advance, it seems likely that non-surgical postoperative refractive adjustments will become available. One example is RxSight’s light-adjustable lens that was FDA-approved in 2017. This lens undergoes predictable changes in curvature when exposed to UV-light, which allows for refractive “touch-ups” after surgery with in-office laser procedures.[24] Studies have shown promising results with 92% of patients within 0.5 D and 99.5% within 1.0 D of the intended refraction after laser adjustment. Uncorrected visual acuity was 20/25 or better in 91.6% of patients.[24] Investigators reported need for adequate pupil dilation for the laser procedure as the most significant problem. There were rare cases of red-tinted vision and color vision abnormalities in patients undergoing light-adjustable lens implantation and there has yet to be sufficient time for long-term data.[24] There was a need for patients to wear UV-blocking sunglasses, even when indoors, with the first generation of the light-adjustable lens, to prevent early refractive shifts. The newer RxSight Light Adjustable Lens with ActivShield mitigates the need for these UV-blocking sunglasses post-operatively.
Additional Resources
References
- ↑ Lagrasta JM, Allemann N,Scapucin L, et al. Clinical results in phacoemulsification using the SRK/T formula. Arq Bras Oftalmol. 2009;72(2):189-193.
- ↑ Cooke DL, Cooke TL. Comparison of 9 intraocular lens power calculation formulas. J Cataract Refract Surg. 2016;42(8):1157-1164.
- ↑ Kane JX, Van Heerden A, Atik A, Petsoglou C. Accuracy of 3 new methods for intraocular lens power selection. J Cataract Refract Surg. 2017;43(3):333-339.
- ↑ Aristodemou P, Knox Cartwright NE, Sparrow JM, Johnston RL. Formula choice: Hoffer Q, Holladay 1, or SRK/T and refractive outcomes in 8108 eyes after cataract surgery with biometry by partial coherence interferometry. J Cataract Refract Surg. 2011;37(1):63-71.
- ↑ Holland E, Lane S, Horn JD, Ernest P, Arleo R, Miller KM. The AcrySof Toric intraocular lens in subjects with cataracts and corneal astigmatism: a randomized, subject-masked, parallel-group, 1-year study. Ophthalmology. 2010;117(11):2104-2111
- ↑ Gale RP, Saldana M, Johnston RL, Zuberbuhler B, McKibbin M. Benchmark standards for refractive outcomes after NHS cataract surgery. Eye (Lond). 2009;23(1):149-152.
- ↑ Lee ES, Lee SY, Jeong SY, et al. Effect of postoperative refractive error on visual acuity and patient satisfaction after implantation of the Array multifocal intraocular lens. J Cataract Refract Surg. 2005;31(10):1960-1965.
- ↑ Jump up to: 8.0 8.1 8.2 8.3 Shalchi Zea. Managing Refractive Surprise. In. Focus: Royal College of Ophthalmologists; 2018.
- ↑ Tsai PS, Dowidar A, Naseri A, McLeod SD. Predicting time to refractive stability after discontinuation of rigid contact lens wear before refractive surgery. J Cataract Refract Surg. 2004;30(11):2290-2294.
- ↑ Hashemi H, Firoozabadi MR, Mehravaran S, Gorouhi F. Corneal stability after discontinued soft contact lens wear. Cont Lens Anterior Eye. 2008;31(3):122-125
- ↑ Cooke and Cooke. Comparison of 9 intraocular lens power calculation formulas. J Cataract Refract Surg 2016 Aug 42(8);1157-64.
- ↑ El-Nafees R, Moawad A, Kishk H, Gaafar W. Intra-ocular lens power calculation in patients with high axial myopia before cataract surgery. Saudi J Ophthalmol. 2010;24(3):77-80.
- ↑ Donoso R, Mura JJ, López M, Papic A. [Emmetropization at cataract surgery. Looking for the best IOL power calculation formula according to the eye length]. Arch Soc Esp Oftalmol. 2003;78(9):477-480.
- ↑ Wan et al. Accuracy and precision of intraocular lens calculations using the new Hill-RBF version 2.0 in eyes with high axial myopia. Am J Ophthalmol. 2019 Sept;205:66-73.
- ↑ Chen C, Xu X, Miao Y, Zheng G, Sun Y. Accuracy of Intraocular Lens Power Formulas Involving 148 Eyes with Long Axial Lengths: A Retrospective Chart-Review Study. J Ophthalmol. 2015;2015:976847.
- ↑ Thebpatiphat N, Hammersmith KM, Rapuano CJ, Ayres BD, Cohen EJ. Cataract surgery in keratoconus. Eye Contact Lens. 2007;33(5):244-246.
- ↑ Wang et al. Accuracy of intraocular lens formulas in eyes with keratoconus. Am J Ophthalmol 2020 Apr;212:26-33.
- ↑ Kelly SP, Jalil A. Wrong intraocular lens implant; learning from reported patient safety incidents. Eye (Lond). 2011;25(6):730-734.
- ↑ Turnbull AMJ, Barrett GD. Using the first-eye prediction error in cataract surgery to refine the refractive outcome of the second eye. J Cataract Refract Surg. 2019;45(9):1239-1245.
- ↑ Jump up to: 20.0 20.1 20.2 20.3 Stephenson M. Refractive Surprises After Cataract Surgery. In: Review of Ophthalmology; 2014.
- ↑ Jump up to: 21.0 21.1 Schallhorn SC, Venter JA, Teenan D, et al. Outcomes of excimer laser enhancements in pseudophakic patients with multifocal intraocular lens. Clin Ophthalmol. 2016;10:765-776.
- ↑ Yeu E. Premium IOLs: Dealing with postop problems. In: Review of Ophthlamology; 2019.
- ↑ Jump up to: 23.0 23.1 Fernández-Buenaga R, Alió JL, Pérez Ardoy AL, et al. Resolving refractive error after cataract surgery: IOL exchange, piggyback lens, or LASIK. J Refract Surg. 2013;29(10):676-683.
- ↑ Jump up to: 24.0 24.1 24.2 Moshirfar M, McCaughey MV, Santiago-Caban L. Corrective Techniques and Future Directions for Treatment of Residual Refractive Error Following Cataract Surgery. Expert Rev Ophthalmol. 2014;9(6):529-537.