All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
Disease Entity
Pythium Keratitis (Pythium insidiosum Keratitis )
Disease
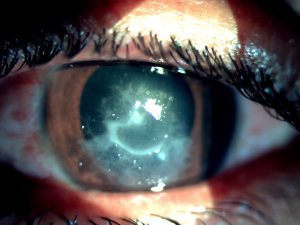
Pythium insidiosum keratitis (PIK) is a rare and vision-threatening keratitis caused by the fungus-like aquatic oomycete Pythium insidiosum. PIK is a relatively uncommon form of keratitis, but its potential to cause blinding manifestations in the eye makes it an important cause. It is most frequently misdiagnosed as fungal keratitis. It belongs to the Genus Pythium, Kingdom Straminipila, Phylum Oomycota, Class Oomycetes, Order Pythiales, and Family Pythiaceae. [1] Pythium insidiosum closely mimics fungal keratitis and hence is also labeled as "parafungus". Pythium recently came into vogue due to its rare clinical and morphological presentation, difficulty in diagnosis by routinely available microbiological methods, high virulence, rapid progression, poor visual prognosis, and high recurrence rate. Clinically, morphologically and microbiologically, it closely mimics fungal keratitis, which makes it a clinical and diagnostic dilemma for clinicians. Pythium lacks ergosterol in its cell wall, making it unresponsive to conventional antifungals, which increases ocular comorbidity in these cases.[2] There is a high possibility that we were routinely missing these cases by wrongly labeling them as fungal keratitis on smearing characteristics or falsely labeling them as unidentified fungus. The hallmark clinical features such as reticular dot infiltrate, tentacles, peripheral guttering, thinning with furrowing, and rapid limbal spread distinguish it from other microbial keratitis (Figure 1). Smearing with Gram, KOH, acridine orange, and IKI-H2SO4 reveals septate or aseptate perpendicular hyphae, closely resembling fungal smear characteristics and making the diagnosis a dilemma. Pythium is picked up culture on blood agar, chocolate agar, potato dextrose agar, or lactophenol cotton blue. It usually appears as dull grey to brown refractile colonies. The confirmatory diagnosis is by zoospore identification by leaf incarnation method.[3]
Currently, culture and polymerase chain reaction (PCR) as labeled as gold standard methods of diagnosis. PIK can also be picked on confocal microscopy and immunohistochemical analysis, but these modalities have limited diagnostic accuracy, are costly, and are unavailable at all centres. PIK still misses established treatment protocols. The currently available medical management modalities are antifungals, antibacterials, and cyanoacrylate glue with bandage contact lenses.[4] Due to the lack of ergosterol, antifungals are no longer recommended as first-line drugs, and antibacterials are the drug of choice. Since Pythium mimics fungus, earlier it was treated with antifungals, but with deeper insights and understanding of its cellular morphology and the absence of ergosterol, antifungals are no longer recommended. In rapidly progressive cases with impending corneal perforation and non-resolving cases, therapeutic keratoplasty with a good 1 mm margin is recommended.[5] In the majority of cases with PIK, the visual outcome is poor. In a large-scale analysis of 71 patients from South India, the authors concluded that antifungals are ineffective against Pythium and TPK is the treatment of choice.[3] Bagga et al., in their analysis, concluded that the minimum inhibitory concertation of antibacterials (topical 0.2% Linezolid and 1% Azithromycin) is more than that of antifungals; hence antibacterials can be labeled as the drug of choice. Is it also believed that the infection was unrecognized in India and Asia subcontinent due to a lack of awareness about identification techniques and no standardized treatment protocols? Systemic Pythiosis has been reported for a long with poor outcomes and high morbidity and mortality rates.[6]
Pythium has added to the burden of microbial keratitis globally. It is an important infection of concern as it doesn't scar easily, requires multiple surgical interventions, and adds to the carbon footprint due to multiple follow-up visits. Gurnani et al. described the novel diagnostic and management protocol to facilitate diagnosis at centres with limited diagnostic facilities and confidently manage the entity. Prompt diagnosis, meticulous management, and regular and timely follow-up are needed to reduce the global burden of Pythium keratitis.[7] The recent reports on Pythium have improved knowledge regarding the variable presentation of the microorganism and have improved diagnostic techniques like zoospore identification by leaf incarnation method and DNA sequencing. Pythium remains a diagnostic and treatment dilemma at most centres across the globe and requires a high index of clinical suspicion and laboratory support to diagnose it.[4]
Epidemiology
Pythium is found in tropical, subtropical, and temperate climates. The majority of the early reports were from Thailand and Israel. Systemic Pythiosis was first reported in 1884 by British Veterinarians. However, it is endemic in Thailand, where the first case was reported in 1985 during a rainy season outbreak. The first case of ocular Pythiosis was reported in 1988, and since then, numerous cases have been reported worldwide. [8] The ocular infection has been commonly reported from Thailand, Australia, China, the United States, Israel, and recently from, India. Despite the omnipresence of this organism, ocular Pythiosis cases have been underdiagnosed and less frequently reported. Besides ocular disease, subcutaneous, vascular, and systemic pythiosis have been documented in the literature.[8][9][10][11] Many studies on Pythium keratitis from southern India have been documented. Two forms of the organism are known: the hyphate form with right-angle branching and broad filaments and the infective form, biflagellate zoospore, in aquatic environments. Since the earlier reports on Pythium keratitis from 1988 to 2010, recently, there has been an upsurge in large-scale prospective and retrospective case series across the globe. Few recent studies have reported more than 50% of cases as students, software professionals, and homemakers.[12]
Pythium has also been reported commonly in farmers and outdoor workers as the animal is found in aquatic rice fields, soil, mud, and brackish water. Studies quoting microorganisms' exact incidence and prevalence are relatively rare in literature. Pythium has also been reported in animals, plants, and horses, and case reports with varied presentations have been reported from Argentina, Brazil, Costa Rica, China, Israel, Australia, India, and Indonesia.[13] In an analysis done during the COVID-19 pandemic, Pythium incidence was reported as rare compared to fungal keratitis. Hasika et al. reported a prevalence of 5.9% of PIK from the South Indian cohort.[3] A rising incidence of PIK has been reported in patients with systemic Pythiosis, such as thalassemia, thrombosis, aneurysms, vasculitis, arterial insufficiency, and paroxysmal nocturnal hemoglobinuria. Vishwakarma et al. reported a high incidence of PIK in males with a higher prevalence among agricultural workers of lower socio-economic strata. Few previous reports have also suggested an association between Pythiosis and exposure to warm freshwater habitats.[14] According to the internal transcribed spacer region or cytochrome oxidase II gene, Pythium insidiosum has been classified into three clades based on geographic location. Clade I (ATH) contains isolates from the United States of America, Class II (BTH) contains isolates from Asia and Australia, and Class III (CTH) is from Thailand and the United States of America.[15]
Etiology
Early reports of pythiosis from veterinary doctors catering to horses led to a hypothesis of the infection in those near horses.[16] However, later, the association was not found to be too strong. Reports from Thailand highlighted the outbreak of Pythium keratitis in the rainy season, and hence link of exposure to aquatic environments was found.[17] An exposure to agricultural aquatic surroundings may be associated however, many studies have shown a large number of patients with a non-agricultural history. Often there might just be a history of exposure to dust particles or foreign bodies falling in the eye. The species is predominantly seen in moist soil, aquatic environment, brackish water, rice, and paddy fields. Pythium is common in rainy outbreaks, especially in Thailand and land flooded for lowland rice production.[18] The most commonly reported specie is Pythium insidiosum, and the other less common one is Pythium aphanidermatum.[19] There are four different forms of human Pythiosis.
Classification
Spectrum of Infection Caused by Pythium
- Vascular Pythiosis - causes arterial occlusions or aneurysms
- Ocular Pythiosis- causes keratitis
- Cutaneous/subcutaneous Pythiosis- causes granulomatous, ulcerating, or cellulitic lesions
- Disseminated form- systemic spread such as cavernous sinus thrombophlebitis[2]
Risk Factors
Exposure to aquatic environments, agricultural areas, soil, and dust are possible risk factors in the development of Pythium keratitis.[4] Working in close proximity to horses is also attributed to some. Cases related to contact lens use have also been reported.[20] Few previous studies also reported Pythium after exposure to dirty water, clay, mud, foreign body fall, ocular trauma, and vegetative matter.[6] Systemic risk factors could be malnourishment, alcoholism, immunosuppression, thalassemia, sickle cell anemia, chronic arterial insufficiency, aplastic anemia, cavernous sinus thrombophlebitis, skin and subcutaneous tissue, aneurysm, thrombosis, vasculitis, and ocular surface surgery.[2] The other common risk factors are swimming with contact lenses, farm animal exposure, and swampy area exposure. A few studies from South India have reported a high incidence of Pythium in housewives, software professionals, students, and people residing in urban locales.[21]
Life cycle of Pythium
A study by Mendoza et al. explained the life cycle of pythium using an in vitro model.[22] They isolated strains of Pythium using equine cases of Pythiosis. This was followed by a demonstration of the asexual reproduction of pythium on plant tissue using an induction medium containing potassium biphosphate and chloride salts of magnesium and calcium. Biflagellate zoospores hence produced were examined under light microscopy for motility. The chemotactic behavior of these spores was assessed towards bits of grass, lily leaves, hair, and biopsied horse hide. Zoospores got attracted to all these tissues and encysted in them, followed by the production of germ tubes. These germ tubes later developed into mycelium and produced sporangia containing biflagellate zoospores. This study highlighted that P. insidiosum requires aquatic plants to complete its life cycle; hence, aquatic and humid habitats may support its propagation.[22]
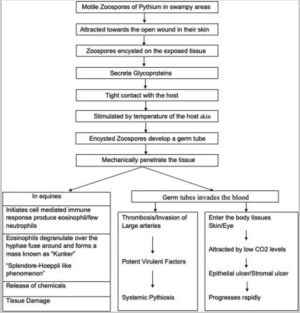
In humans, infection is acquired through contact with open skin wounds. The motile zoospore of Pythium is usually seen in dirty and swampy areas. The zoospore is attracted to the open wound site in the skin. The zoospore work by encysting on the exposed tissue. The zoospore, in tight contact with the skin surface, secrete glycoproteins. The encysted zoospore develops a germ tube on exposure to high temperature on coming in contact with the skin.[23] The germ tube mechanically penetrates the skin and causes infection; in equines, it alters the cell-mediated immune response and produces eosinophils and neutrophils. The eosinophils degranulate around the hyphae, fuse together, and form a mass known as "Kunker." This is labeled as a "Splendore Hoeppli" like phenomenon. This leads to the release of chemicals and subsequent tissue damage. In humans, when the germ tube invades the blood vessels, it leads to thrombosis of large arteries, releasing chemicals and subsequent systemic pythiosis. Another mechanism is when the germ tube invades the eye and skin tissue and is attracted by low carbon dioxide levels; it leads to epithelial defect and stromal infiltration, which progresses rapidly (Figure 3).[24]
Histopathology
One of the major reasons pythium is misdiagnosed as fungi is that it resembles the hyphate organism in light microscopy. Classically described to be seen as broad, branching, aseptate to the sparsely septate organism.[25] However, pythium lacks ergosterol and, on the contrary, contains cellulose and β-glucan in its cell wall. Histopathological findings from corneal tissue infected with pythium reveal diffuse destruction of the corneal stroma.[26] The inflamed tissue consisted of polymorphs mixed with eosinophils, plasma cells, and debris. Few histiocytes and multinucleated giant cells can be present in the posterior stromal exudates and along Descemet's membrane. Granulomatous inflammation can be noted in 15% of the cases.[27] Also interesting to note is that although Pythium is known to cause vascular disease, it spares the vessels of the eye.
Classical Pathological Features of Pythium Insidiosum Keratitis
A large number of authors have described virulence factors and susceptibility to the hosts. Pythium is unresponsive to conventional antifungals due to a lack of ergosterol in the cell wall and clinically, morphologically, and microbiologically closely mimics fungal keratitis.[4] There is a rapid progression of the ulcer despite the host's immune response, which is unclear in the literature. One of the previous studies studied the pathogenetic mechanism of the formation of zoospores. Zoospores are formed after 1 hour of induction, and encyst rapidly produces germ tubes within 24 hours.[28] This results in the formation of a large quantity of mycelium and a fulminant course of the disease. Krajaejun et al. studied the Pythium insidiosum genes involved in oxidative stress response, copper and zinc superoxide dismutase, thioredoxin, and glutaredoxin. The authors identified 16 putative proteins and sequence tags having similarities with the virulence factors of the fungus. The same authors also identified calmodulin, heat shock proteins, and transcription factors expressed through sequence tags which were responsible for pathogen growth and thermal adaptation inside the host.[29] Another study identified beta-D glucan and Pythium insidiosum IgG antibodies as potential rescue markers for vascular pythiosis.[30] Lelievre described that thalassemia patients are susceptible to Pythium insidiosum due to the expression of the gene coding ferrochelatase, an enzyme required for heme biosynthesis. The iron in the nonbound state is attached to microbial heme and stored for further metabolic use. In 2005, another study described proteases as a potential virulent factor causing human keratitis.[31]
Diagnosis
Diagnosis of Pythium keratitis is often delayed due to its rarity. Therefore it becomes very important to know when clinical suspicion exists. Routine corneal scraping should be done with a sterile instrument (blade, kimura’s spatula, or 26G needle) under topical anesthesia at the slit lamp. The microbiologist should be specifically informed to look for Pythium otherwise it can go undetected. Pythium can be grown on potato dextrose agar, Sabouraud dextrose agar, and chocolate agar. Other modalities like PCR and confocal microscopy have also been successful in diagnosis.[2]
History
Several reports have highlighted that pythium keratitis is more commonly seen in non-agricultural workers as opposed to fungal keratitis.[12] A vague history of exposure to dust or an insignificant foreign body may be present. Nevertheless, some patients of Pythium keratitis might have an account of exposure to aquatic environments like agricultural water pools or rainwater. Some reports also suggest the precedence of vegetative trauma or rarely trauma with a horsetail.[32] The occupational history of working in animal homes, specially stables, should be asked for. The history is usually of short duration, and presentation lag can be between 1 day to 3 weeks, as commonly described in the literature. There can be a previous history of stick injury, dust fall, clay injury, and contact lens wear. In sporadic cases, the history of trauma is missing.[4]
Symptoms
Usually, patients present with an acute red eye with the presence of a white spot on the cornea.[3] Severe photophobia and foreign body sensation may accompany. Pain in the affected eye is commonly found in varied intensities depending on the extent of involvement. Time taken from the onset of symptoms to hospital presentation may vary from 2 to almost as long as 60 days. [12] The patient usually presents with pain, redness, watering, discharge, photophobia, and reduced visual acuity, which is common for microbial keratitis.[3]
Physical Examination
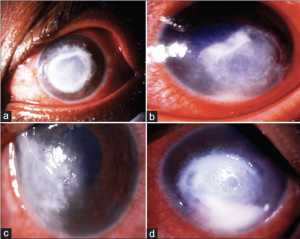
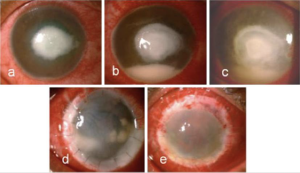
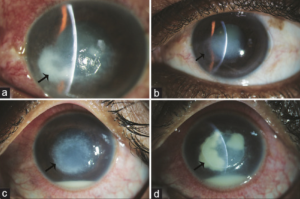
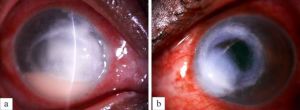
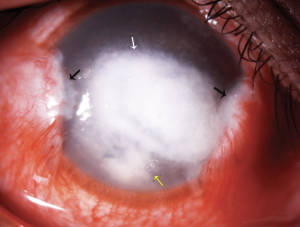
Vision may range from 6/6 to as poor as light perception depending on the severity of keratitis.[33] The ulcer classically appears as having a dense gray-white necrotic surface with feathery margins and flocculent debris overlying. Multiple mid-stromal “tentacle-like infiltrates' and “subepithelial expanding dot-like infiltrates" have been described.[3][34] The other classical features include stromal infiltration with hyphated edges and peripheral furrowing.[35] The exact pathogenesis behind these pinhead lesions is unclear but they are claimed to be due to the organism itself rather than stromal inflammatory response and expand in a reticular pattern. Radial keratoneuritis can be present.[36] Some patients might have peripheral furrow-like lesions. Associated stromal edema and Descemet's folds may be present. Hypopyon and endothelial plaques may accompany severe ulcers. Advanced cases may have corneal melts and perforation. The typical course of Pythium keratitis is prolonged and marred by its not-so-easily scarring quality. Aggressive progression may lead to scleral and limbal involvement too. Ocular involvement apart from keratitis can be in the form of a rapidly progressive periorbital or orbital cellulitis reported in children. The clinical features which distinguish PIK from fungal keratitis are patchy reticular dots like sub-epithelial and stromal infiltrates, multifocal lesions, cotton wool-shaped stromal infiltrate with hyphated edges, peripheral guttering with furrowing, early limbal involvement, tentacular projections, and rapid corneal thinning (Figure 4, 5, 6,7).[37]
Overlapping Clinical Features
Multiple clinical findings in Pythium insidiosum overlap with the clinical findings of various microorganisms.
Fungal Keratitis
The overlapping clinical findings can be an epithelial defect, subepithelial and stromal infiltrate with feathery margins, satellite lesion, endothelial exudates, ring infiltrate, descemetocele, perforation, hypopyon, and gradual spread towards the limbus.[32]
Bacterial Keratitis
In bacterial keratitis, there are more symptoms than signs; there can be associated greenish discharge, epithelial defect, subepithelial and stromal infiltrate, corneal melt, cheesy white suppurative infiltrate, corneal abscess, endothelial exudates, hypopyon, and perforation.[31]
Acanthamoeba Keratitis
Satellite lesions, focal corneal infiltrates, ring infiltrate, stromal suppuration, and radial keratoneuritis.[36]
Atypical Mycobacterial Keratitis
Epithelial defect, dry greyish white stromal infiltrate, and stromal edema, Descemet membrane folds, crack windshield-like appearance.[34]
Peripheral Ulcerative Keratitis
Peripheral thinning with guttering, crescentic stromal infiltrate, stromal cellular reaction, stromal edema, and corneal perforation.[38]
Viral Keratitis
Ring infiltrate, stromal infiltrate, and dot infiltrate appear like subepithelial infiltrate, corneal thinning, melt, and perforation.[14]
Pediatric Pythium Keratitis
The clinical features of pediatric PIK resemble that of adult PIK. Treating a pediatric PIK aims to eliminate the infective focus, prevent stromal melt and perforation, safeguard the vision, and prevent amblyopia. One of the previous reports labeled radial keratoneuritus as a feature of pediatric PIK. Only 4 cases of pediatric PIK have been documented in the literature.[26][35] [39][40] The scarcity of literature makes it difficult to pinpoint a particular clinical feature as a typical pediatric PIK clinical feature.
Badenoch et al. reported PIK in a three-year-old girl from Australia who presented with a diffuse central corneal infiltrate, stromal thinning, keratic precipitates, and anterior chamber hypopyon closely mimicking fungal keratitis. The child had a history of swimming in the swimming pool.[39] Pythium was confirmed by culture, and treatment was started with polyhexamethylene biguanide and voriconazole for five days which was unsuccessful. Five days later, the child was taken up for keratoplasty. The eye was salvaged with functional vision after 14 months of treatment. The pythium diagnosis was confirmed by rRNA gene sequencing.
He et al. reported PIK in a 7-year-old child from Hainan province in south China who developed a suppurative nasal peripheral white stromal ulcer with diffuse infiltration along with multiple radial keratoneuritis after coming from a forest. Culture, smear, and confocal imaging were suggested for fungal hyphae. The patient was treated with natamycin, fluconazole, and voriconazole for one month was unsuccessful, and the child underwent therapeutic penetrating keratoplasty with anterior vitrectomy. There was graft infection again after one day, and anterior chamber irrigation was performed with 0.02% fluconazole and Amphotericin B solution. Finally, the globe was salvaged, and there was no recurrence. The diagnosis was confirmed on PCR to be Pythium insidiosum.[26]
Chatterjee and Aggarwal reported PIK in a 7 year-old boy with a dense central oval shaped dry looking gray white stromal infiltrate with feathery margins extending to the posterior stromal and having an overlying epithelial defect and tentacular projections. The margins of the ulcers appear, and there is hypopyon in the anterior chamber. After the initial microscopic examination, a diagnosis of fungal keratitis was made, and the child was treated with natamycin every half hourly, atropine three times daily, along with oral analgesics. Ten days later, as the child didn't improve, he was treated with additional 1% voriconazole. Despite this, the ulcer progressed, and rescraping was performed. Further, the direct microscopic examination was suggestive of broad aseptate hyaline hyphae with ribbon-like folds at right angle bends. Then a presumed diagnosis of PIK was made, and the patient was treated with 1% azithromycin eye drops every hour, 1% voriconazole, and 1% atropine eye drop three times per day along with oral Azithromycin 250 mg once daily for three days and oral analgesics. In view of corneal thinning, cyanoacrylate glue, and bandage contact lens was applied to prevent perforation. After thirteen weeks of treatment, the cornea was completely epithelized, and the globe was salvaged. Later the child was awaiting penetrating keratoplasty.[40]
Gurnani et al. salvaged a child who was 9 years and had PIK. The child had a dry-looking ulcer with a feathery margin and associated stromal edema following a stick injury. The child's visual acuity of 6/12 deteriorated to HM+ within five days of treatment. The initial smear examination was suggestive of fungal keratitis, and before the culture results, the patient was treated with 5% natamycin and 1% Itraconazole. Since the progression of the ulcer, a differential diagnosis of PIK was made, which was confirmed with the blood agar culture. After the culture results, the patient was managed with 0.2% Linezolid and 1% Azithromycin hourly. Since there was rapid progression and corneal melt, the patient was managed with cyanoacrylate glue and bandage contact lens. On follow-up, BCVA improved to 6/12.[35]
Gurnani and Kaur Severity Grading of Pythium Insidiosum Keratitis[7]
| S.No | Characteritics | Mild | Moderate | Severe |
|---|---|---|---|---|
| 1 | Size of Infiltrate | < 2 mm | 2x2- 4x4 mm | >4x4 mm |
| 2 | Infiltrate depth | Superficial 1/3, 33% depth | Up to mid-stroma (33-50%) | Mid-stroma and beyond (>50%) |
| 3 | Depth of ulcer | Superficial 1/3 33% depth | Up to mid-stroma, 33–50% depth | Mid stroma and beyond, >50% depth |
| 4 | Scleral involvement | Absent | Absent | Present |
| 5 | Corneal perforation | Absent | Unlikely | Impending or present |
| 6 | Presence of hallmark features of Pythium | Absent | Reticular dot infiltrates, tentacles | Peripheral furrowing, limbal spread, early corneal melt |
| 7 | Hypopyon | Absent | It may or may not be seen | Present in the majority of cases |
Associations of Systemic Pythiosis
- Thalassemia[18]
- Thalassemia Hemoglobinopathy syndrome[41]
- Paroxysmal Nocturnal Haemoglobinuria[42]
- Chronic Arterial Insufficiency[42]
- Aplastic Anaemia[43]
- Thrombosis[44]
- Cavernous Sinus Thrombophlebitis[44]
- Aneurysms[45]
- Vasculitis[46]
- Skin, subcutaneous tissue ulceration, and granulomas[1]
Diagnostic Procedures
Lab Diagnosis
As we all know, Pythium keratitis is a rare entity requiring a high index of clinical suspicion for diagnosis. Besides clinical clues, the diagnosis is supplemented by laboratory investigations. Clinicians, microbiologists, and pathologists need to rule out atypical microbial keratitis as these keratitis has poorer outcomes. To establish a diagnosis, the treating clinician should need to perform corneal scraping with smear, culture, button culture post keratoplasty, and culture of the eviscerated button to establish the diagnosis.[47] The other important modalities are the leaf incarnation method to culture zoospores, polymerase chain reaction, and confocal microscopy.[22][48][49]
Corneal Smear
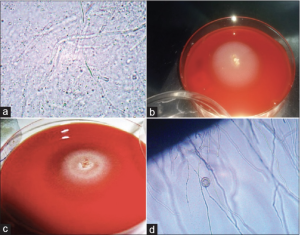
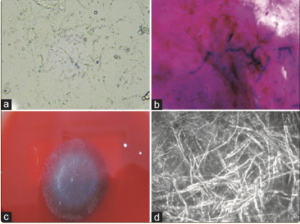
Corneal scraping, as done routinely for all corneal ulcers, should be done. The presence of relatively broader hyphae with sparse or no septations and right-angled branching is seen on KOH mount (Figure 8a, 9a) and Gram staining (Figure 9b). This often misleads the examiner in favor of fungal etiologies. Hence laboratory demonstration of zoospore production remains confirmatory. But this is fraught with limitations of longer diagnosis time and unavailability of growth media (broth culture or water containing antibiotic agent and human hair).[27][50]
The corneal scraping should be performed under aseptic precautions under topical anesthesia by using 0.5% proparacaine with the help of either a Kimura spatula, Bark-Parker blade, 26 G needle, or 15 number blade. The specimen should be taken from the base and edges of the ulcer for maximum yield and studied under a microscope. Two scrapings should be obtained, one for Gram stain and another for 10% potassium hydroxide. Further, three scrapings should be obtained for culture on blood agar (BA), chocolate agar (CA), and potato dextrose agar (PDA). Smear morphology reveals typical long aseptate or sparsely septate hyaline hyphae with numerous vesicles. The hyphae can have collapsed walls and perpendicular lateral branches; the dimensions are 3-10 mm and can sometimes be as large as 10 mm.[27]
In some cases, ribbon-like folding of Pythium hyphae is also observed. These are the morphological characteristics of Pythium filaments. In contrast, the fungal filaments are broad, sparsely septate, and branch at various angles. Since it is difficult to differentiate the smear characteristics of fungus from Pythium, many novel stains have recently been available to improve the diagnosis. The other stains available are calcofluor white (CFW), acridine orange, lactophenol cotton blue (LPCB), trypan blue, and potassium iodide sulfuric acid (IKI-H2SO4).[27] One of the recent studies used a combination of 10% KOH-CFW for increasing the diagnostic yield and found that the sensitivity was between 79.3% and 96.5%, and specificity was above 93% for detecting Pythium.[51] But this required fluorescent microscopy and a microbiologist to assess the images, which was one of the limitations. Another study used trypan blue to study the hyphal characteristics without any special microscope and found a sensitivity of over 75% and a specificity of 68%. Trypan blue is helpful in low-resource settings such as rural backgrounds without advanced diagnostic modalities.[52] The recent diagnostic stain IKI-H2SO4 help stain Pythium filaments, and few studies have shown specificity close to 100%. The sensitivity and specificity of various stains concerning the culture results of PIK. Different combinations of the above stains and clinical suspicion help in the early detection of PIK. Other listed stains are Haematoxylin and Eosin (H and E), Periodic Acid- Schiff (PAS) stain, and help to pick up Pythium filaments from formalin-fixed, paraffin-embedded (FFPE) samples.[53]
H and E and PAS stain reveal necrotic granulomas around the hyphae. After staining with H and E, the Pythium filaments turn pale pink to ghost-like filaments. Additionally, the slides can be stained with 0.5% and 1% periodic acid for 0.5, 2, 3 and 5 minutes, and Schiff's reagent should be applied without any modification for 10 minutes. The filaments that turn pink are labeled positive; when there is no staining, they are labeled negative. The Pythium filaments with GMS appear brown, and the stroma may show a greenish hue. The filaments show varied morphology on GMS, such as septate or aseptate, broad or narrow, short or long, and may appear obtuse to perpendicular. The IKI-H2SO4 is a cost-effective, simple, sensitive, and specific stain for diagnosis of oomycete of Pythium. The hyphae which stain blue/bluish black are labeled as positive, and which appear as yellow or yellowish brown appear as labeled as negative.[27]
Culture
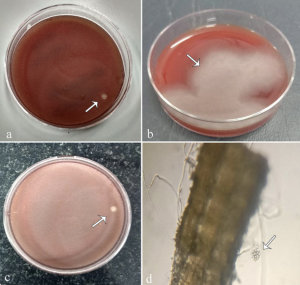
The scraping sample is placed on the culture plate as C-shaped streaking and cultured overnight. Culture can be done using blood agar (Figure 8b, 8c, 9c, 10a, 10b), Potato dextrose agar, Sabouraud agar without antibiotics, yeast extract–peptone–dextrose (YPD), or chocolate agar (Figure 8c). Flat, feathery, light brown colonies with filiform margins can be seen on blood agar. On potato dextrose agar, pythium colonies are seen as adherent flat, smooth, opaque, and white-yellowish, with filiform margins.[1] The blood agar plates are incubated at 37°C in a 5% CO2 incubator, and PDA is incubated at 27°C, and the colonies are observed for macroscopic morphology. The Pythium colonies appear flat, feathery, cotton wool-like, partially submerged, colorless to light brown small hair-like projections with fine radiations at the edges. The incubation plate can also appear as hyaline or submerged colony morphology. Pythium colonies can be obtained on any form of nutritional agar. Grass leaves with water culture can also induce zoospore formation, which is diagnostic of Pythium, but the process is time-consuming and takes approximately 11 days for identification and delay the initiation of targeted antimicrobial therapy.[22] One of the recent and novel techniques described for identifying Pythium species in the early stage of culture is Matrix-assisted laser desorption ionization-time of flight mass spectrometry (MALDI-TOF).[54]
Leaf Incarnation Method for Zoospore Identification
The gold standard for zoospore identification is a culture of suspected isolate on blood agar or chocolate agar, non-nutrient agar on autoclaved carnation plant leave, which are approximately 2x2 mm in size, and induction media. The growth is rapid, and the zoospore formation and vesicles can be noticed as late as 6-12 hours (Figure 8d, 10d)[22]
Serological Testing
Serology-based tests that detect antigens and antibodies can also pick up pythium. These include hemagglutination assays (HA), ELISA, immunodiffusion (ID) testing, and immunochromatography (ICT) for the detection of Pythiosis. Immunoperoxidase staining, ELISA, and ICT have high sensitivity and specificity. These serological testing methods have excellent diagnostic utility in systemic pythiosis but have limited utility in PIK cases, as there is no systemic antibody response in keratitis patients.[2]
Molecular Testing of Pythium
Polymerase Chain Reaction
Next to a positive culture, PCR is considered the gold standard as it identifies the specie specific sequence of the DNA. The sample for PCR is either obtained as corneal scraping or the growth on a culture plate. In PCR, rDNA-ITS (internal transcribed spacer) regions are targeted. PCR as a diagnostic modality for Pythium detection has rapidly evolved. The diagnostic ability of nested PCR is extremely good, with around 90% sensitivity and 100% specificity.[55][56]
The novel duplex PCR targets 18S rRNA and ITS region, which shows 100% specificity and 91% sensitivity. Another vogue technique is LAMP (loop-mediated isothermal amplification) PCR which has 100% sensitivity and 98% specificity. Real-time PCR also targets a protein named exo-1,3 beta-glucanase with 100% sensitivity and specificity apart from a short turnaround time of 7.5 hours. PCR has also been used for various population-based studies of Pythium, and target molecules such as COX-II, ELI025, exo-1,3-β-glucanase gene, and AFLP (amplified fragment length polymorphism) have also been used to study Pythium species.[57][58]
Imaging
Confocal Microscopy
The overlap of staining characteristics with fungal keratitis, delay in culture diagnosis, and definitive need for zoospore diagnosis had made researchers to search for alternatives to diagnose Pythium with the help of imaging techniques. Serological testing has made rapid strides in systemic Pythiosis diagnosis, but its role in PIK has been limited. The use of confocal microscopy has been described for the diagnosis of PIK, but the confocal imaging characteristics overlap with that of fungi, and it's difficult to delineate Pythium from fungi on confocal microscopy. The Pythium filaments appear as beaded, thin hyper reflective branching structures with a mean branching angle of 78.6° and length varying from 90 to 400 um. Confocal microscopy has a role in the detection of early recurrence of Pythium. In vivo, confocal microscopy (IVCM) cannot differentiate Pythium from fungus or Nocardia. Multiple refractile filaments often showing right-angled branching and arranging in alphabetical patterns like X, Y, or S can be demonstrated on confocal microscopy (Figure 9d).[2][24][49]
Other Investigations
Immunoperoxidase Staining
Immunoperoxidase staining using various Pythium insidiosum antibodies has been documented to have higher sensitivity and specificity than routine histopathological stains. In an analysis, Kosirurkvongs et al. compared the nested PCR with culture and immunostaining and found PCR to be the most sensitive.[59]
Spectrometry
Matrix-assisted laser desorption ionization-time of flight mass spectrometry (MALDI-TOF MS) is a recent analytical tool to identify Pythium proteins by assessing the crude proteins using a main spectral profile (MSP) which is then compared against a reference database for P. insidiosum.[59] A new technology by the name of matrix-assisted laser desorption ionization-time of flight mass spectrometry (MALDI-TOF) has shown success in the quick diagnosis of Pythium.[60]
Differential Diagnosis
Due to its close morphological resemblance to fungal hyphae, PIK is often misdiagnosed and treated as fungal keratitis until signs of non-resolution are apparent. Also, the resemblance in both patients' historical patterns might confuse the clinician. Classic tentacle lesions with subepithelial pinhead infiltrates can clinch the diagnosis of Pythium keratitis. Further staining patterns with PAS and other immunohistochemical methods can differentiate between the two entities. Another closely resembling etiology is Acanthamoeba keratitis, which also presents with a similar history of exposure to aquatic environments. The differentiating factor is essentially a ring-shaped ulcer with radial perineuritis and diagnostic double-walled cysts in microscopy in acanthamoeba patients.[1][2]
The close differentials of Pythium insidiosum keratitis
- Fungal keratitis- Fusarium, Aspergillus, Curvalaria, Alternaria[32]
- Bacterial keratitis- Nocardia, Staphylococcus, Streptococcus, Pseudomonas[31]
- Atypical Mycobacterial keratitis[34]
- Acanthamoeba keratitis[36]
- Necrotizing viral keratitis[14]
- Viral keratitis with secondary bacterial infiltrate[20]
- Peripheral ulcerative keratitis[38]
- Marginal keratitis[38]
- Mooren's ulcer[38]
Management
The prognosis of Pythium keratitis is not very good, firstly due to delay in diagnosis and secondly because of the dearth of management options. The remission of disease by medical therapy alone is questionable. As already discussed, P. insidiosum is refractory to antifungal treatment. However, few cases have been seen to respond to 1% itraconazole.[3] In vitro, studies have shown varied success with antimicrobials acting on protein synthesis inhibition. In particular, macrolides and tetracyclines have shown synergistic activity. Increased awareness, improved understanding of the pathogenetic mechanism, varied clinical presentation, and understanding microbiological features have led to more straightforward diagnosis; however, management remains a challenge. There is no standard gold treatment for PIK; however, antimicrobial susceptibility testing studies have favored antibiotics as the first line drugs.[6]
Medical Management
Antifungal Drugs
Pythium was categorized under fungal ulcer earlier; hence antifungals such as 5% Natamycin, 1% Voriconazole, 1% Itraconazole, and 2% Ketoconazole were considered the first-line drugs. With the current understanding of Pythium's cell wall structure, antifungals have minimal activity in Pythium as the cell wall lacks ergosterol.[3][5] Bagga et al., in their randomized trial, concluded that the minimal inhibitory concentration (MIC) of antibacterials is more compared to antifungals in the treatment of PIK; hence antibacterials are the first-line drugs.[6] They also concluded the limited success of antifungals. Recent case reports and large-scale studies have also labeled the limited role of antifungals. Hasika et al. also concluded that TPK is the treatment of choice for PIK, and antifungals have a limited role.[3] Though provoking paper by Gurnani et al. showed that antifungals still have a role to play in PIK. Gurnani et al. proposed that smearing Pythium hyphae completely mimics fungal hyphae, and it becomes difficult to pinpoint the diagnosis before culture results are available. Hence, before culture results are available (usually five days) and if clinical features mimic fungal keratitis, antifungals should be given until Pythium is diagnosed on culture. Once culture results suggest PIK, antifungal drugs can be substituted. There can be a mixed infection in some instances; hence, antifungals and antibiotics can be continued in these cases.[7][24] Suppose hallmark clinical features of Pythium, such as reticular dot infiltrate, tentacular projections, and guttering, are present. In that case, antibacterials with antifungals should be given because fungal cannot be ruled out until culture-negative results. If cases appear like PIK clinically but culture negative for Pythium, they should be labeled as fungal keratitis and must be started on antifungal drugs. The Pythium cell wall comprises cellulose, chitin, and a minimal beta-glucan quantity. Caspofungin inhibits beta glucan synthase and is more effective than echinocandins. However, the effect is transient and statics, and the results were not encouraging.[22]
Antibacterial Drugs
In vitro susceptibility studies and in vivo rabbit models of Pythium have recommended antibacterials such as 0.2% Linezolid and 1% Azithromycin as the first-line drugs for managing PIK.[61] Studies with broth microdilution, Etest, and disk diffusion methods following the CLSI guidelines have shown minocycline, tigecycline, Azithromycin, and clarithromycin to be more effective than antifungals.[62] Linezolid can be prepared from IV preparation or crushed capsules mixed with normal saline. The drug regimen should be hourly for the first week, and the drugs can be tapered based on the clinical response. Azithromycin can also be used as eye ointment, and oral Azithromycin can also be given 500 mg per day for two weeks. These patients should be followed up to assess the response, and clinical resolution is denoted by a reduction in cellular density of surrounding stroma, reduction in tentacles, blunting of ulcer margin, and reduced density of the infiltrate. The peripheral guttering and deep vascularization should be closely observed to prevent perforation. Gurnani et al., in their retrospective analysis, proposed that when the ulcer size was less than 4x4 mm and not involving a visual axis, monotherapy of linezolid was preferred.[4] However, a combination of Linezolid and Azithromycin was given when the measure was more than 4x4 mm, involving mid-stroma and beyond and affecting vision. Recent large-scale studies from India, especially South India, have used antibacterials before TPK, and the success rate has been found to be as high as 25-30% with conservative medical management. Antibiotics have changed and shaped the management protocol in PIK. The other commonly available and tried antibiotics in PIK are tetracyclines, lincosamides, streptogramins, phenols, aminoglycosides, macrolides, oxazolidinones, and glycylcyclines. Other combinations like that of topical and oral Azithromycin and topical voriconazole, a variety of topical minocycline ointment four times daily, hourly chloramphenicol drops and oral linezolid 1200 mg BD , and a combination of oral doxycycline and clindamycin and topical Azithromycin have also been used with partial success. Β-glucan inhibitors like caspofungin have been tried but are inconclusive. Another agent, mefenoxam, an RNA synthesis inhibitor, has shown some promise in animal experiments. Debulking of infectious load by scraping can be beneficial, but caution should be executed for the risk of perforation in severe cases.[5]
Adjuvant Therapy
Anti-glaucoma Medications
Antiglaucoma medications in the form of beta-blockers (e.g., Timolol, Betaxolol, Carteolol) or adrenergic agonists (e.g., brimonidine, dipivefrine) can be started in cases with secondary glaucoma or when there is hypopyon in the anterior chamber. These drugs cause a reduction in intraocular pressure, reduce microcystic epithelial edema and give pain relief to the patient. There can also be a rise in IOP secondary to inflammation; hence antiglaucoma medications play a central role in the management.[32]
Cycloplegic Medications
Cycloplegic medications such as atropine, homatropine, and cyclopentolate are required to relieve pain from ciliary spasms, prevent the formation of posterior synechiae, break already formed synechiae, and also break down the blood-aqueous barrier. Cycloplegic medication plays a vital role in pain relief and preventing occlusio or seclusion pupillae formation.[63]
Anti-inflammatory Medications
Anti-inflammatory medications such as diclofenac, paracetamol, Ibuprofen, indomethacin, ketoprofen, and flurbiprofen are essential oral anti-inflammatory drugs to control inflammation and pain in PIK cases and even post keratoplasty cases.[35]
Surgical Management
The majority of case reports and studies from Thailand, China, and Australia have embarked on surgical treatment as the mainstay of treatment for Pythium keratitis. Cases with multiple recurrences, endophthalmitis, or panophthalmitis may require enucleation or evisceration.
Therapeutic Keratoplasty (TPK)
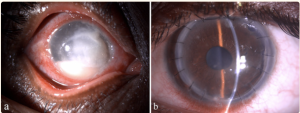
Pythium is a rapidly proliferating microorganism with high virulence, high recurrence rate, and has limited response to existing antimicrobial drugs. Hence, in most cases, medical treatment alone is insufficient to control or eradicate the infection. These patients require early and aggressive therapeutic keratoplasty (TPK), antibacterials, and adjuvant drugs.[3] TPK in PIK differs slightly from bacterial, fungal, and acanthamoeba keratitis. In PIK, early TPK, usually after 10-14 days of initial presentation, is warranted. The host margin trephination should be 1-1.5 mm more significant than the infiltrate, and a thorough anterior chamber wash with intracameral antibacterials is required for a better outcome (Figure 11).[4][14] The indications of TPK in PIK include early stromal melt, severe corneal thinning, descemetocele, impending perforation, perforated corneal ulcer, rapid limbal spread, scleral involvement, and non-resolving endoexudates. The recurrence rate is very high in these cases, as Gurnani et al.[4] and Agarwal et al.[12] reported. Hasika et al. reported that TPK is the mainstay of treatment of PIK. TPK, in its early stages, is considered the gold standard treatment of PIK.[64]
Optical Penetrating Keratoplasty (OPK)
As per conventional norms, in cases that have undergone TPK, OPK should be attempted after one year of TPK. But it is not always possible to wait for one year in these cases; hence OPK can be attempted even after 6-8 months of quiescence once the eye is infection free. OPK helps in the visual rehabilitation of these patients and gives vision to the blind and needy, especially the one-eyed patients. The host trephination in OPK should be kept between 7-7.5 mm and the donor size 0.5 mm greater than the hosts. In cases having cataract, a triple procedure OPK + Cataract extraction with IOL implantation should be performed as a visual rehabilitative procedure.[4]
Descemet Stripping Endothelial Keratoplasty (DSEK)
In cases with endothelial rejection post-OPK, DSEK is an excellent procedure for visual rehabilitation. DSEK is a lamellar transplant, hence preserving the anatomical integrity, preventing complications of open sky procedure, and has less incidence of graft rejection with early visual rehabilitation.[4]
Enucleation and Evisceration
Non-resolving cases, multiple recurrences, scleral abscesses, endophthalmitis, and panophthalmitis are some of the important indications for enucleation and evisceration in PIK cases. Rapid virulence, rapid proliferation, early limbal spread, guttering, and perforation have made the management of these cases extremely difficult. Puangsricharern et al., in their retrospective analysis, showed that out of 25 patients, 15 globes were removed. The globe removal group patients had more satellite lesions and multifocal and total corneal infiltrates. Evisceration or enucleation may be required in severe infection with diffuse scleritis or endophthalmitis.[64][65]
Adjunctive Treatment Modalities
Cryotherapy with Ethanol
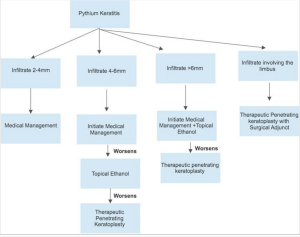
Adjunctive modalities that have been used to decrease recurrence have been tried with limited success. These include single-cycle cryotherapy with a retinal probe at the graft host junction. In patients with scleral involvement, 99.9% absolute alcohol should be applied beyond cryotherapy marks extending to the posterior limit of the infected sclera for 20 seconds. The mechanism for cryotherapy is dehydration and ischemic infarction of the cells. There is also an accumulation of toxic metabolites within the cell walls, which leads to cellular destruction. Ethanol leads to apoptosis, promotes cell lysis, reduces the proliferation of cells, and reduces the cellular life span. In one of the previous studies by Agarwal et al., cryotherapy was tried with ethanol in six PIK cases having an anterior chamber and scleral involvement. Out of the 6, only one patient had a recurrence, thus showing the efficacy of ethanol as an adjunctive treatment modality.[66] Figure 12 depicts the flowchart for management of PIK based on infiltrate and by employing topical ethanol as an adjunct to prevent recurrence in PIK.
Cyanoacrylate Glue with Bandage Contact Lens
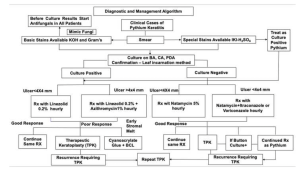
Cyanoacrylate glue is required in cases with early stromal melt, significant corneal thinning, impending perforation, descemetocele, corneal perforation, and peripheral guttering patients. Cyanoacrylate glue plugs the perforation site and prevents hypotony and other complications of the shallow anterior chamber. As reported in the literature, cyanoacrylate glue has antibacterial properties and acts synergistically with first-line antibacterials such as Linezolid and Azithromycin. Thus cyanoacrylate glue has the dual benefit of restoring anatomical integrity and having a therapeutic effect on ulcer healing. Glue, along with BCL, has an additive effect on ulcer healing.[35] Gurnani et al. successfully managed a 9 year-old boy with significant corneal thinning with cyanoacrylate glue with BCL, thus proving the efficacy of this adjunctive treatment modality.[35] Chatterjee and Agrawal also managed a pediatric patient with a non-resolving ulcer having significant stromal thinning with cyanoacrylate glue and BCL and found excellent outcomes after 14 weeks of prolonged treatment .[40] Figure 13 depicts the detailed diagnostic and management algorithm for PIK.
Vaccine Containing Pythium Insidiosum Antigen
Isolated reports of vaccines containing P. Insidiosum antigen (PIA) used to decrease post-keratoplasty infection recurrence have been noted. But the success of this therapy is not documented and needs more extensive study validation.[43]
Role of Immunotherapy
Immunotherapy with Pythium insidiosum immunotherapy (PIA), along with a combination of medical therapy and surgery, has also been suggested as an adjunctive treatment for managing PIK. PIA has shown an acceptable safety profile, but the role in PIK is still questionable in a retrospective analysis of 10 eyes of 10 patients. All the cases of PIK were diagnosed by culture or PCR. Three doses of PIAI were injected into the patients at two-week intervals in all the patients. Out of 10 cases, 8 had hypopyon, and the infiltrate diameter ranged from 5.2 mm to total corneal ulcer. Nine cases underwent TPK, and enucleation was done in 1 patient. One case required a repeat TPK, and two globes were salvaged; two cases received voriconazole via eyedrops and intracameral injection. None of the cases received antibacterials. Only three eyes could be saved. Hence, it was concluded that PIAI does not effectively treat PIK.[67]
Monitoring and Follow-up
Daily monitoring of these cases is warranted for timely intervention. The rapidly progressive and aggressive nature of the pathogen can lead to a sudden worsening in a matter of a few days. There is a more than 50% recurrence reported even after timely surgical intervention by penetrating keratoplasty and as early as within two weeks.[49][17] Careful evaluation of graft clarity and particularly graft host junction should be done. Any new infiltrate or anterior chamber exudation should be considered serious. These patients may need prompt regrafting with additive therapies in the form of cryotherapy or alcohol treatment as described. Severe cases, suspected orbital infections, and large scleral involvement should undergo imaging to rule out chances of cavernous sinus thrombosis.[44]
Postoperative Graft Management
Post TPK, in preoperative culture-proven PIK cases, anti-pythium drugs (Linezolid and Azithromycin) should be continued starting from 6-8 times days, and the patient should be followed closely on days 1, 5, 14, and so on as per the clinical condition. In cases with early recurrence, hourly antimicrobial therapy should be instituted, and clinical response should be noted. Gurnani et al. gave a conceptual approach for managing PIK cases.[4] They suggested that if preoperative and postoperative culture reports are adverse, this is suggestive of limited to no microorganism load; hence, topical steroids can be started after two weeks of anti-pythium drug therapy provided there are no signs of graft infection or anterior chamber recurrence. In patients with preoperative or postoperative culture positivity, waiting for at least three weeks before starting topical steroids is wiser. If both preoperative and postoperative culture results are positive, then it is wiser to wait at least 3-4 weeks before starting steroids. Steroids should be started based on the clinical condition of the graft under close vigilance. In the first week, topical steroids can be activated three times per day, and if the response is good with no recurrence of infection, topical steroids can be stepped up four times per day. Topical steroids should be supplemented with anti-pythium drugs for an initial couple of weeks. The patients should be followed up closely, and counseling should be stressed. Topical steroids of choice are 0.1% dexamethasone and 1% prednisolone. Adjuvant drugs such as homatropine and timolol also help relieve pain and prevent uveitis sequelae and secondary glaucoma. Post TPK, there is a high incidence of cataracts and glaucoma which should be managed accordingly.[1][4]
Complications
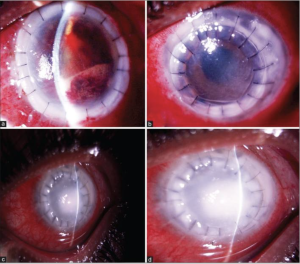
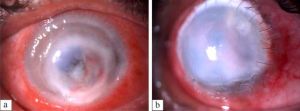
A few of the complications of PIK are shown in Figure 14, 15. The complications can be divided as
Preoperative[4]
- Corneal melt
- Corneal thinning
- Descematocoele
- Impending perforation
- Corneal perforation
- Secondary glaucoma
- Uncontrolled uveitis
- Complicated cataract
- Peripheral corneal guttering
- Panuveitis
- Endophthalmitis
- Panophthalmitis
- Wound leak
- Eccentric graft
- Epithelial defect
- Descemet membrane detachment
- Iridodialysis
- Lens expulsion
- Nucleus drop
- Vitreous prolapse
- Expulsive choroidal haemorrhage
- Hyphema
- Wound leak
- Hypotony
- Suture infection
- Suture infiltrate
- Suture vascularization
- Epithelial defect
- Graft infection
- Anterior chamber exudate
- Graft failure
- Vitreous wick syndrome
- Vitreous touch syndrome
- Hyphema
- Blood clot
- Fibrinous membrane
- Vitritis
- Cataract
- Secondary glaucoma
- Choroidal detachment
- Retinal detachment
- Panuveitis
- Endophthalmitis
- Panophthalmitis
- Scleritis
- Scleral abscess
- Toxic anterior segment syndrome
- Phthisis bulbi
Prognosis
The majority of literature available on PIK has reported poor prognostic outcomes even after TPK leading to regraft, graft infection, enucleation, evisceration, and Phthisis bulbi.[64] The prognosis in these cases is governed by the time of presentation, time lag, ulcer size, depth, the virulence of microorganisms, lack of availability of diagnostic modalities, misuse of steroids, delay in initiation of treatment, misdiagnosis, and wrongly instituted antimicrobial therapy. The recent advances in the diagnosis and treatment of Pythium keratitis have made way for better results. Large case series have evaluated the prognosis of pythium and report that the once severely morbid ocular disease has improved outcomes with timely intervention.[4] The rate of radical procedures like evisceration and enucleation has decreased to almost 15% as opposed to 90% in historical times. Still, the high recurrence in grafts is worrisome. Advances in molecular technology may lead to the identification of the genome of P. insidiosum, and hence better therapeutic strategies could further increase the survival of grafts in these patients. Superficial corneal infiltrates, if treated aggressively with antibacterials, scar early and have good outcomes. PIK with full-thickness infiltrates, endoexudates, rapid limbal spread, peripheral furrowing, and hypopyon requires early and aggressive TPK and the prognosis. Patients with complications such as panophthalmitis, endophthalmitis, retinal detachment, graft infection, and choroidal detachment had extremely guarded prognoses. In these cases, timely TPK with good margin clearance can have good anatomical and functional outcomes. Meticulously performed TPK leads to graft failure with corneal scarring and can be managed with a good OPK at a later date.[68]
Future Directions and Management Options
A large number of drugs and different treatment options have been tried in human Pythiosis, and these drugs have shown excellent responses in these cases. These drugs have the potential to become future treatment options in PIK. The proposed treatment options are multi-drug therapy and drug combinations, biogenic silver products, natural plant compounds, plant extracts, copper acetate, bacterial isolates, and miltefosine (a drug for Leishmaniasis).[2] These drugs have shown promising efficacy in PIK, but more extensive clinical trials are required to prove the efficacy. The antibacterials which have shown good efficacy against PIK are oxazolidinones (linezolid), macrolides (Azithromycin, clarithromycin), aminoglycosides (neomycin, streptomycin), phenicol's (chloramphenicol), tetracyclines (tigecycline and minocycline), lincosamides (clindamycin), and streptogramins (quinupristin dalfopristin). PIK has also shown a good response to glycylcyclines. Immunotherapy is an upcoming modality of treatment wherein in vitro culture PI antigens are injected into the patient.[5] The other important drugs which have shown excellent efficacy are Nitrofurantoin, Mupirocin, and Xanthyletin. Nitrofurantoin is the drug of choice for urinary tract infections which inhibits mycelial growth of PIK when in vitro susceptibility tests were used. Mupirocin also inhibits PI isolates with a minimum inhibitory concentration of less than. 4 ug/ml. Xanthyletin, a coumarin derivative from green plants, has also been shown to have an anti-Pythium effect by impacting the protein composition of the cell wall. Streptomyces has also been scaled as a biocontrol agent against Pythium. Another potential future treatment option for PIK is photodynamic therapy. Amorolfine hydrochloride, a novel antifungal agent, inhibits ergosterol synthesis and changes the membrane permeability. They have also been shown to have in vitro inhibitory activity against PI. Disulfiram has also been employed as an anti-Pythium agent and has demonstrated maximum activity at MIC of 8-32 mg/liters. Nitric oxide antibiotics have also been explored as therapeutic targets against human Pythiosis. Pseudomonas stutzeri and Klebsiella pneumoniae metabolites have also been shown to inhibit Pythium.[2][5]
Additional Suggested Literature for Reference
[1] https://link.springer.com/article/10.1007/s40135-022-00302-7, Cao B, Gonugunta VT, Radhakrishnan N, Lalitha P, Gurnani B, Kaur K, Iyer G, Agarwal S, Srinivasan B, Keenan JD, Prajna NV. Outcomes of Pythium keratitis: a meta-analysis of individual patient data. Curr Ophthalmol Rep. 2022 Dec;10(4):198-208. doi: 10.1007/s40135-022-00302-7. Epub 2022 Nov 10. PMID: 37250102; PMCID: PMC10211475.
[2] https://journals.lww.com/ijo/Fulltext/2023/05000/Outcomes_of_therapeutic_penetrating_keratoplasty.34.aspx, Kate A, Thigale U, Ponnapati LP, Chaudhary S, Vishwakarma P, Sharma S, Bagga B. Outcomes of therapeutic penetrating keratoplasty in Pythium insidiosum keratitis managed with a combination of antibiotics. Indian J Ophthalmol. 2023 May;71(5):1868-1874. doi: 10.4103/IJO.IJO_2862_22. PMID: 37203046.
[3] https://www.dovepress.com/predicting-prognosis-based-on-regional-prevalence-ulcer-morphology-and-peer-reviewed-fulltext-article-OPTH, Gurnani B, Kaur K. Predicting Prognosis Based on Regional Prevalence, Ulcer Morphology and Treatment Strategy in Vision-Threatening Pythium insidiosum Keratitis. Clin Ophthalmol. 2023 May 5;17:1307-1314. doi: 10.2147/OPTH.S412274. PMID: 37181081; PMCID: PMC10167989.
[4] https://journals.lww.com/corneajrnl/Abstract/9900/Randomized_Double_Masked_Placebo_Controlled_Trial.243.aspx, Tanna V, Bagga B, Sharma S, Ahirwar LK, Kate A, Mohamed A, Joseph J. Randomized Double-Masked Placebo-Controlled Trial for the Management of Pythium Keratitis: Combination of Antibiotics Versus Monotherapy. Cornea. 2023 Feb 14. doi: 10.1097/ICO.0000000000003251. Epub ahead of print. PMID: 36796011.
[5] https://journals.lww.com/ijo/Fulltext/2023/02000/Clinical_differentiation_of_keratitis_due_to.40.aspx, D'cruz RP, Mohamed A, Das S. Clinical differentiation of keratitis due to fungus and Pythium: A photographic survey. Indian J Ophthalmol. 2023 Feb;71(2):510-514. doi: 10.4103/ijo.IJO_913_22. PMID: 36727350; PMCID: PMC10228981.
https://journals.lww.com/ijo/Fulltext/2022/10000/Clinical_differentiation_of_Pythium_keratitis_from.20.aspx, Chatterjee S, Agrawal D, Gomase SN. Clinical differentiation of Pythium keratitis from fungal keratitis and development of a scoring system. Indian J Ophthalmol. 2022 Oct;70(10):3515-3521. doi: 10.4103/ijo.IJO_870_22. PMID: 36190038; PMCID: PMC9789832.
[6] https://www.sciencedirect.com/science/article/abs/pii/S025508572200144X?via%3Dihub, Sahoo S, Mitra S, Mittal R, Behera HS, Das S. Use of different stains for microscopic evaluation for the diagnosis of Pythium keratitis. Indian J Med Microbiol. 2022 Oct-Dec;40(4):521-524. doi: 10.1016/j.ijmmb.2022.08.003. Epub 2022 Aug 27. PMID: 36041948.
[7] https://www.tandfonline.com/doi/abs/10.1080/08820538.2022.2116287?journalCode=isio20, Vishwakarma P, Bagga B. Pythium insidiosum keratitis: Review of literature of 5 years' clinical experience at a tertiary eye care center. Semin Ophthalmol. 2023 Feb;38(2):190-200. doi: 10.1080/08820538.2022.2116287. Epub 2022 Aug 29. PMID: 36036721.
[8] https://www.ajtmh.org/view/journals/tpmd/107/1/article-p110.xml, Thanathanee O, Bhoomibunchoo C, Anutarapongpan O, Suwan-Apichon O, Charoensuk K, Chindamporn A. Role of Immunotherapy in Pythium insidiosum Keratitis. Am J Trop Med Hyg. 2022 Jun 6;107(1):110-112. doi: 10.4269/ajtmh.22-0015. PMID: 35895358; PMCID: PMC9294668.
[9] https://link.springer.com/article/10.1007/s40123-022-00542-7, Gurnani B, Kaur K, Agarwal S, Lalgudi VG, Shekhawat NS, Venugopal A, Tripathy K, Srinivasan B, Iyer G, Gubert J. Pythium insidiosum Keratitis: Past, Present, and Future. Ophthalmol Ther. 2022 Oct;11(5):1629-1653. doi: 10.1007/s40123-022-00542-7. Epub 2022 Jul 5. PMID: 35788551; PMCID: PMC9255487.
References
- ↑ 1.0 1.1 1.2 1.3 1.4 Gurnani B, Kaur K. Pythium Keratitis. 2022 Dec 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan–. PMID: 34424645.
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 2.6 2.7 2.8 Gurnani B, Kaur K, Agarwal S, Lalgudi VG, Shekhawat NS, Venugopal A, Tripathy K, Srinivasan B, Iyer G, Gubert J. Pythium insidiosum Keratitis: Past, Present, and Future. Ophthalmol Ther. 2022 Oct;11(5):1629-1653. doi: 10.1007/s40123-022-00542-7. Epub 2022 Jul 5. PMID: 35788551; PMCID: PMC9255487.
- ↑ 3.0 3.1 3.2 3.3 3.4 3.5 3.6 3.7 3.8 3.9 Hasika R, Lalitha P, Radhakrishnan N, Rameshkumar G, Prajna NV, Srinivasan M. Pythium keratitis in South India: Incidence, clinical profile, management, and treatment recommendation. Indian J Ophthalmol. 2019 Jan;67(1):42-47. doi: 10.4103/ijo.IJO_445_18. PMID: 30574890; PMCID: PMC6324135.
- ↑ 4.00 4.01 4.02 4.03 4.04 4.05 4.06 4.07 4.08 4.09 4.10 4.11 4.12 4.13 4.14 4.15 Gurnani B, Christy J, Narayana S, Rajkumar P, Kaur K, Gubert J. Retrospective multifactorial analysis of Pythium keratitis and review of literature. Indian J Ophthalmol. 2021 May;69(5):1095-1101. doi: 10.4103/ijo.IJO_1808_20. PMID: 33913840; PMCID: PMC8186601.
- ↑ 5.0 5.1 5.2 5.3 5.4 Gurnani B, Kaur K, Kumar T. Commentary: Current concepts, recent updates, and future treatment options for Pythium insidiosum keratitis. Indian J Ophthalmol. 2023 May;71(5):1874-1876. doi: 10.4103/IJO.IJO_80_23. PMID: 37203047.
- ↑ 6.0 6.1 6.2 6.3 6.4 Bagga B, Sharma S, Madhuri Guda SJ, Nagpal R, Joseph J, Manjulatha K, Mohamed A, Garg P. Leap forward in the treatment of Pythium insidiosum keratitis. Br J Ophthalmol. 2018 Dec;102(12):1629-1633. doi: 10.1136/bjophthalmol-2017-311360. Epub 2018 Mar 15. PMID: 29545414.
- ↑ 7.0 7.1 7.2 Gurnani B, Kaur K. Predicting Prognosis Based on Regional Prevalence, Ulcer Morphology and Treatment Strategy in Vision-Threatening Pythium insidiosum Keratitis. Clin Ophthalmol. 2023 May 5;17:1307-1314. doi: 10.2147/OPTH.S412274. PMID: 37181081; PMCID: PMC10167989.
- ↑ 8.0 8.1 Virgile R, Perry HD, Pardanani B, et al. A human infectious corneal ulcer caused by Pythium insidiosum. Cornea. 1993;12:81–83
- ↑ Triscott JA, Weedon D, Cabana E. Human subcutaneous pythiosis. J Cutan Pathol 1993;20:267-71
- ↑ Sudjaritruk T, Sirisanthana V. Successful treatment of a child with vascular pythiosis. BMC Infect Dis 2011;11:33
- ↑ Reanpang T, Orrapin S, Orrapin S, Arworn S, Kattipatanapong T, Srisuwan T, et al. Vascular pythiosis of the lower extremity in Northern Thailand: Ten years' experience. Int J Low Extrem Wounds 2015;14:245-50
- ↑ 12.0 12.1 12.2 12.3 12.4 12.5 Agarwal S, Iyer G, Srinivasan B, Benurwar S, Agarwal M, Narayanan N, Lakshmipathy M, Radhika N, Rajagopal R, Krishnakumar S, K LT. Clinical profile, risk factors and outcome of medical, surgical and adjunct interventions in patients with Pythiuminsidiosum keratitis. Br J Ophthalmol. 2019 Mar;103(3):296-300. doi: 10.1136/bjophthalmol-2017-311804. Epub 2018 Sep 11. PMID: 30206158.
- ↑ Nguyen D, Vilela R, Miraglia BM, Vilela G, Jasem-Alali N, Rohn R, Glass R, Hansen RD, Mendoza L. Geographic distribution of Pythium insidiosum infections in the United States. J Am Vet Med Assoc. 2021 Dec 27;260(5):530-534. doi: 10.2460/javma.20.10.0595. PMID: 34968184.
- ↑ 14.0 14.1 14.2 14.3 Vishwakarma P, Mohanty A, Kaur A, Das S, Priyadarshini SR, Mitra S, Mittal R, Sahu SK. Pythium keratitis: Clinical profile, laboratory diagnosis, treatment, and histopathology features post-treatment at a tertiary eye care center in Eastern India. Indian J Ophthalmol. 2021 Jun;69(6):1544-1552. doi: 10.4103/ijo.IJO_2356_20. PMID: 34011738; PMCID: PMC8302330.
- ↑ Appavu SP, Prajna L, Rajapandian SGK. Genotyping and phylogenetic analysis of Pythium insidiosum causing human corneal ulcer. Med Mycol. 2020 Feb 1;58(2):211-218. doi: 10.1093/mmy/myz044. PMID: 31073609.
- ↑ Smith F. The pathology of bursattee. Vet J 1884;19:16-7
- ↑ 17.0 17.1 Thanathanee O, Enkvetchakul O, Rangsin R, Waraasawapati S, Samerpitak K, Suwan-Apichon O, et al. The outbreak of Pythium keratitis during rainy season: A case series. Cornea 2013;32:199-204
- ↑ 18.0 18.1 Imwidthaya P. Human pythiosis in Thailand. Postgrad Med J. 1994 Aug;70(826):558-60. doi: 10.1136/pgmj.70.826.558. PMID: 7937448; PMCID: PMC2397701.
- ↑ Calvano TP, Blatz PJ, Vento TJ, Wickes BL, Sutton DA, Thompson EH, White CE, Renz EM, Hospenthal DR. Pythium aphanidermatum infection following combat trauma. J Clin Microbiol. 2011 Oct;49(10):3710-3. doi: 10.1128/JCM.01209-11. Epub 2011 Aug 3. PMID: 21813724; PMCID: PMC3187319.
- ↑ 20.0 20.1 Tanhehco TY, Stacy RC, Mendoza L, Durand ML, Jakobiec FA, Colby KA. Pythium insidiosum keratitis in Israel. Eye Contact Lens. 2011;37(2):96-98.
- ↑ Agarwal S, Iyer G, Srinivasan B, Agarwal M, Panchalam Sampath Kumar S, Therese LK. Clinical profile of pythium keratitis: perioperative measures to reduce risk of recurrence. Br J Ophthalmol. 2018 Feb;102(2):153-157. doi: 10.1136/bjophthalmol-2017-310604. Epub 2017 Sep 13. PMID: 28903964.
- ↑ 22.0 22.1 22.2 22.3 22.4 22.5 Mendoza L, Hernandez F, Ajello L. Life cycle of the human and animal oomycete pathogen Pythium insidiosum [published correction appears in J Clin Microbiol 1994 Jan;32(1):276]. J Clin Microbiol. 1993;31(11):2967-2973. doi:10.1128/JCM.31.11.2967-2973.1993
- ↑ Gaastra W, Lipman LJ, De Cock AW, Exel TK, Pegge RB, Scheurwater J, Vilela R, Mendoza L. Pythium insidiosum: an overview. Vet Microbiol. 2010 Nov 20;146(1-2):1-16. doi: 10.1016/j.vetmic.2010.07.019. Epub 2010 Jul 24. PMID: 20800978.
- ↑ 24.0 24.1 24.2 Gurnani B, Kaur K, Venugopal A, Srinivasan B, Bagga B, Iyer G, Christy J, Prajna L, Vanathi M, Garg P, Narayana S, Agarwal S, Sahu S. Pythium insidiosum keratitis - A review. Indian J Ophthalmol. 2022 Apr;70(4):1107-1120. doi: 10.4103/ijo.IJO_1534_21. PMID: 35325996; PMCID: PMC9240499.
- ↑ Thomas PA. Current perspectives on ophthalmic mycoses. Clin Microbiol Rev. 2003 Oct;16(4):730-97. doi: 10.1128/CMR.16.4.730-797.2003. PMID: 14557297; PMCID: PMC207127.
- ↑ 26.0 26.1 26.2 He H, Liu H, Chen X, Wu J, He M, Zhong X. Diagnosis and Treatment of Pythium Insidiosum Corneal Ulcer in a Chinese Child: A Case Report and Literature Review. Am J Case Rep. 2016 Dec 27;17:982-988. doi: 10.12659/ajcr.901158. PMID: 28025573; PMCID: PMC5207016.
- ↑ 27.0 27.1 27.2 27.3 27.4 Mittal, Ruchi MD, DNB*,†; Jena, Shipra K. MSc*; Desai, Alisha MS†; Agarwal, Sunil MD‡ Pythium Insidiosum Keratitis: Histopathology and Rapid Novel Diagnostic Staining Technique, Cornea: September 2017 - Volume 36 - Issue 9 - p 1124-1132
- ↑ Chaiprasert A, Samerpitak K, Wanachiwanawin W, Thasnakorn P. Induction of zoospore formation in Thai isolates of Pythium insidiosum. Mycoses. 1990 Jun;33(6):317-23. doi: 10.1111/myc.1990.33.6.317. PMID: 2259373.
- ↑ Krajaejun T, Kittichotirat W, Patumcharoenpol P, Rujirawat T, Lohnoo T, Yingyong W. Genome data of four Pythium insidiosum strains from the phylogenetically-distinct clades I, II, and III. BMC Res Notes. 2021 May 21;14(1):197. doi: 10.1186/s13104-021-05610-y. PMID: 34020710; PMCID: PMC8139067.
- ↑ Worasilchai N, Permpalung N, Chongsathidkiet P, Leelahavanichkul A, Mendoza AL, Palaga T, Reantragoon R, Finkelman M, Sutcharitchan P, Chindamporn A. Monitoring Anti-Pythium insidiosum IgG Antibodies and (1→3)-β-d-Glucan in Vascular Pythiosis. J Clin Microbiol. 2018 Jul 26;56(8):e00610-18. doi: 10.1128/JCM.00610-18. PMID: 29848566; PMCID: PMC6062813.
- ↑ 31.0 31.1 31.2 Lelievre L, Borderie V, Garcia-Hermoso D, Brignier AC, Sterkers M, Chaumeil C, Lortholary O, Lanternier F. Imported pythium insidiosum keratitis after a swim in Thailand by a contact lens-wearing traveler. Am J Trop Med Hyg. 2015 Feb;92(2):270-3. doi: 10.4269/ajtmh.14-0380. Epub 2014 Dec 22. PMID: 25535313; PMCID: PMC4347328.
- ↑ 32.0 32.1 32.2 32.3 Gurnani B, Christy J, Kaur K, Moutappa F, Gubert J. Successful Management of Pythium insidiosum Keratitis Masquerading as Dematiaceous Fungal Keratitis in an Immunosuppressed Asian Male. Ocul Immunol Inflamm. 2023 Feb 22:1-4. doi: 10.1080/09273948.2023.2179495. Epub ahead of print. PMID: 36812410.
- ↑ Aggarwal D, Mitra S, Kalra P, Bagga B, Mishra D, Takkar B. Case Report: Poor Outcome Despite Aggressive Management in Pythium insidiosum Endophthalmitis. Am J Trop Med Hyg. 2022 Dec 12;108(1):27-30. doi: 10.4269/ajtmh.22-0441. PMID: 36509056; PMCID: PMC9833092.
- ↑ 34.0 34.1 34.2 Ramappa M, Nagpal R, Sharma S, Chaurasia S. Successful Medical Management of Presumptive Pythium insidiosum Keratitis. Cornea. 2017;36(4):511-514.
- ↑ 35.0 35.1 35.2 35.3 35.4 35.5 Gurnani B, Narayana S, Christy J, Rajkumar P, Kaur K, Gubert J. Successful management of pediatric pythium insidiosum keratitis with cyanoacrylate glue, linezolid, and azithromycin: Rare case report. Eur J Ophthalmol. 2021 Mar 28:11206721211006564. doi: 10.1177/11206721211006564. Epub ahead of print. PMID: 33779337
- ↑ 36.0 36.1 36.2 Raghavan A, Bellamkonda P, Mendoza L, Rammohan R. Pythium insidiosum and Acanthamoeba keratitis in a contact lens user. BMJ Case Rep. 2018 Dec 3;11(1):bcr2018226386. doi: 10.1136/bcr-2018-226386. PMID: 30567163; PMCID: PMC6301630.
- ↑ Puangsricharern V, Chotikkakamthorn P, Tulvatana W, Kittipibul T, Chantaren P, Reinprayoon U, Kasetsuwan N, Satitpitakul V, Worasilchai N, Chindamporn A. Clinical Characteristics, Histopathology, and Treatment Outcomes of Pythium Keratitis: A Retrospective Cohort Study. Clin Ophthalmol. 2021 Apr 23;15:1691-1701. doi: 10.2147/OPTH.S303721. PMID: 33935486; PMCID: PMC8080432.
- ↑ 38.0 38.1 38.2 38.3 Kate A, Bagga B, Ahirwar LK, Mishra DK, Sharma S. Unusual Presentation of Pythium Keratitis as Peripheral Ulcerative Keratitis: Clinical Dilemma. Ocul Immunol Inflamm. 2022 Oct-Nov;30(7-8):2023-2026. doi: 10.1080/09273948.2021.1952276. Epub 2021 Aug 19. PMID: 34410210.
- ↑ 39.0 39.1 Badenoch PR, Mills RA, Chang JH, Sadlon TA, Klebe S, Coster DJ. Pythium insidiosum keratitis in an Australian child. Clin Exp Ophthalmol. 2009 Nov;37(8):806-9. doi: 10.1111/j.1442-9071.2009.02135.x. PMID: 19878227.
- ↑ 40.0 40.1 40.2 Chatterjee S, Agrawal D. Azithromycin in the Management of Pythium insidiosum Keratitis. Cornea. 2018 Feb;37(2):e8-e9. doi: 10.1097/ICO.0000000000001419. PMID: 29095755.
- ↑ Sathapatayavongs B, Leelachaikul P, Prachaktam R, Atichartakarn V, Sriphojanart S, Trairatvorakul P, Jirasiritham S, Nontasut S, Eurvilaichit C, Flegel T. Human pythiosis associated with thalassemia hemoglobinopathy syndrome. J Infect Dis. 1989 Feb;159(2):274-80. doi: 10.1093/infdis/159.2.274. PMID: 2644370.
- ↑ 42.0 42.1 Chitasombat MN, Jongkhajornpong P, Lekhanont K, Krajaejun T. Recent update in diagnosis and treatment of human pythiosis. PeerJ. 2020 Feb 20;8:e8555. doi: 10.7717/peerj.8555. PMID: 32117626; PMCID: PMC7036273.
- ↑ 43.0 43.1 Wanachiwanawin W, Mendoza L, Visuthisakchai S, Mutsikapan P, Sathapatayavongs B, Chaiprasert A, Suwanagool P, Manuskiatti W, Ruangsetakit C, Ajello L. Efficacy of immunotherapy using antigens of Pythium insidiosum in the treatment of vascular pythiosis in humans. Vaccine. 2004 Sep 9;22(27-28):3613-21. doi: 10.1016/j.vaccine.2004.03.031. PMID: 15315840.
- ↑ 44.0 44.1 44.2 Rathi A, Chakrabarti A, Agarwal T, Pushker N, Patil M, Kamble H, Titiyal JS, Mohan R, Kashyap S, Sharma S, Sen S, Satpathy G, Sharma N. Pythium Keratitis Leading to Fatal Cavernous Sinus Thrombophlebitis. Cornea. 2018 Apr;37(4):519-522. doi: 10.1097/ICO.0000000000001504. PMID: 29319595.
- ↑ Chitasombat MN, Petchkum P, Horsirimanont S, Sornmayura P, Chindamporn A, Krajaejun T. Vascular pythiosis of carotid artery with meningitis and cerebral septic emboli: A case report and literature review. Med Mycol Case Rep. 2018 May 7;21:57-62. doi: 10.1016/j.mmcr.2018.05.003. PMID: 30013898; PMCID: PMC6022254.
- ↑ Khunkhet S, Rattanakaemakorn P, Rajatanavin N. Pythiosis presenting with digital gangrene and subcutaneous nodules mimicking medium vessel vasculitis. JAAD Case Rep. 2015 Nov 5;1(6):399-402. doi: 10.1016/j.jdcr.2015.09.005. PMID: 27051792; PMCID: PMC4809401.
- ↑ Sahoo S, Mitra S, Mittal R, Behera HS, Das S. Use of different stains for microscopic evaluation for the diagnosis of Pythium keratitis. Indian J Med Microbiol. 2022 Oct-Dec;40(4):521-524. doi: 10.1016/j.ijmmb.2022.08.003. Epub 2022 Aug 27. PMID: 36041948.
- ↑ Kulandai LT, Lakshmipathy D, Sargunam J. Novel Duplex Polymerase Chain Reaction for the Rapid Detection of Pythium insidiosum Directly From Corneal Specimens of Patients With Ocular Pythiosis. Cornea. 2020 Jun;39(6):775-778. doi: 10.1097/ICO.0000000000002284. PMID: 32118672.
- ↑ 49.0 49.1 49.2 Anutarapongpan O, Thanathanee O, Worrawitchawong J, Suwan-Apichon O. Role of Confocal Microscopy in the Diagnosis of Pythium insidiosum Keratitis. Cornea. 2018 Feb;37(2):156-161. doi: 10.1097/ICO.0000000000001466. PMID: 29176453.
- ↑ Gurnani B, Kaur K. Comment on: Sensitivity and specificity of potassium hydroxide and calcofluor white stain to differentiate between fungal and Pythium filaments in corneal scrapings from patients of Pythium keratitis. Indian J Ophthalmol. 2022 Jun;70(6):2204. doi: 10.4103/ijo.IJO_345_22. PMID: 35648022; PMCID: PMC9359274.
- ↑ Bagga B, Vishwakarma P, Sharma S, Jospeh J, Mitra S, Mohamed A. Sensitivity and specificity of potassium hydroxide and calcofluor white stain to differentiate between fungal and Pythium filaments in corneal scrapings from patients of Pythium keratitis. Indian J Ophthalmol. 2022 Feb;70(2):542-545. doi: 10.4103/ijo.IJO_1880_21. PMID: 35086234; PMCID: PMC9023980.
- ↑ Sharma S, Rathi VM, Murthy SI, Garg P, Sharma S. Application of Trypan Blue Stain in the Microbiological Diagnosis of Infectious Keratitis-A Case Series. Cornea. 2021 Dec 1;40(12):1624-1628. doi: 10.1097/ICO.0000000000002725. PMID: 33935235.
- ↑ Elshafie NO, Hanlon J, Malkawi M, Sayedahmed EE, Guptill LF, Jones-Hall YL, Santos AP. Nested PCR Detection of Pythium sp. from Formalin-Fixed, Paraffin-Embedded Canine Tissue Sections. Vet Sci. 2022 Aug 19;9(8):444. doi: 10.3390/vetsci9080444. PMID: 36006359; PMCID: PMC9412607.
- ↑ Mani R, Vilela R, Kettler N, Chilvers MI, Mendoza L. Identification of Pythium insidiosum complex by matrix-assisted laser desorption ionization-time of flight mass spectrometry. J Med Microbiol. 2019 Apr;68(4):574-584. doi: 10.1099/jmm.0.000941. Epub 2019 Feb 8. PMID: 30735118.
- ↑ Thongsri Y, Wonglakorn L, Chaiprasert A, et al. Evaluation for the clinical diagnosis of Pythium insidiosum using a single-tube nested PCR. Mycopathologia. 2013;176:369–76.
- ↑ Botton SA, Pereira DI, Costa MM, et al. Identification of Pythium insidiosum by nested PCR in cutaneous lesions of Brazilian horses and rabbits. Curr Microbiol. 2011;62:1225–29.
- ↑ Kosrirukvongs P, Chaiprasert A, Uiprasertkul M, et al. Evaluation of nested pcr technique for detection of Pythium insidiosum in pathological specimens from patients with suspected fungal keratitis. Southeast Asian J Trop Med Public Health. 2014;45:167–73
- ↑ Salipante SJ, Hoogestraat DR, SenGupta DJ, et al. Molecular diagnosis of subcutaneous Pythium insidiosum infection by use of PCR screening and DNA sequencing. J Clin Microbiol. 2012;50:1480–83.
- ↑ 59.0 59.1 Keeratijarut A, Lohnoo T, Yingyong W, et al. Detection of the oomycete Pythium insidiosum by real-time PCR targeting the gene coding for exo-1,3-beta-glucanase. J Med Microbiol. 2015;64:971–77
- ↑ Singhal N, Kumar M, Kanaujia PK, Virdi JS. MALDI-TOF mass spectrometry: an emerging technology for microbial identification and diagnosis. Front Microbiol. 2015 Aug 5;6:791. doi: 10.3389/fmicb.2015.00791. PMID: 26300860; PMCID: PMC4525378.
- ↑ Ahirwar LK, Kalra P, Sharma S, Mohamed A, Mittal R, Das S, Bagga B. Linezolid shows high safety and efficacy in the treatment of Pythium insidiosum keratitis in a rabbit model. Exp Eye Res. 2021 Jan;202:108345. doi: 10.1016/j.exer.2020.108345. Epub 2020 Nov 3. PMID: 33157127.
- ↑ Loreto ES, Tondolo JS, Pilotto MB, Alves SH, Santurio JM. New insights into the in vitro susceptibility of Pythium insidiosum. Antimicrob Agents Chemother. 2014 Dec;58(12):7534-7. doi: 10.1128/AAC.02680-13. Epub 2014 Sep 15. PMID: 25223997; PMCID: PMC4249561.
- ↑ Cabrera-Aguas M, Khoo P, Watson SL. Infectious keratitis: A review. Clin Exp Ophthalmol. 2022 Jul;50(5):543-562. doi: 10.1111/ceo.14113. Epub 2022 Jun 3. PMID: 35610943; PMCID: PMC9542356.
- ↑ 64.0 64.1 64.2 Puangsricharern V, Chotikkakamthorn P, Tulvatana W, Kittipibul T, Chantaren P, Reinprayoon U, Kasetsuwan N, Satitpitakul V, Worasilchai N, Chindamporn A. Clinical Characteristics, Histopathology, and Treatment Outcomes of Pythium Keratitis: A Retrospective Cohort Study. Clin Ophthalmol. 2021 Apr 23;15:1691-1701. doi: 10.2147/OPTH.S303721. PMID: 33935486; PMCID: PMC8080432.
- ↑ Kunavisarut S, Nimvorapan T, Methasiri S. Pythium corneal ulcer in Ramathibodi Hospital. J Med Assoc Thai. 2003 Apr;86(4):338-42. PMID: 12757078.
- ↑ Agarwal S, Srinivasan B, Janakiraman N, Therese LK, S K, Patel N, V T, Iyer G. Role of Topical Ethanol in the Treatment of Pythium insidiosum Keratitis-A Proof of Concept. Cornea. 2020 Sep;39(9):1102-1107. doi: 10.1097/ICO.0000000000002370. PMID: 32541188.
- ↑ Thanathanee O, Bhoomibunchoo C, Anutarapongpan O, Suwan-Apichon O, Charoensuk K, Chindamporn A. Role of Immunotherapy in Pythium insidiosum Keratitis. Am J Trop Med Hyg. 2022 Jun 6;107(1):110-112. doi: 10.4269/ajtmh.22-0015. PMID: 35895358; PMCID: PMC9294668.
- ↑ Permpalung N, Worasilchai N, Manothummetha K, Torvorapanit P, Ratanawongphaibul K, Chuleerarux N, Plongla R, Chindamporn A. Clinical outcomes in ocular pythiosis patients treated with a combination therapy protocol in Thailand: A prospective study. Med Mycol. 2019 Nov 1;57(8):923-928. doi: 10.1093/mmy/myz013. PMID: 30805615.