Pupillary Abnormalities After Glaucoma Tube Shunt Surgery
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
Glaucoma
Introduction and Epidemiology
Glaucoma is a leading cause of blindness and visual impairment both globally and nationally. It is estimated that in 2020, close to 75 million people worldwide will suffer from the condition; this figure is expected to grow to 110 million people by 2040.[1] Given the prevalence of the disease and its devastating consequences, glaucoma and its diagnosis, pathogenesis, and treatment have become major areas of investigation for researchers.
Types and Pathophysiology
While numerous types and subtypes of glaucoma exist, it can broadly be divided into open angle glaucoma and angle-closure glaucoma.[2][3] Primary open angle glaucoma (POAG) is a chronic ocular condition in which the angle between the surface of the iris and the cornea is open, which is the normal healthy state. In open angle glaucoma, there is increased resistance to aqueous outflow at the level of the trabecular meshwork; this increased resistance causes an increase in upstream pressure.[2][3] Conversely, primary angle-closure glaucoma (PACG) can present acutely or chronically and features a mechanical obstruction (generally by the iris or pupil) that prevents aqueous outflow through the angle between the iris and the cornea.[2][4][3] Both conditions can cause an increase in intraocular pressure (IOP) and cause subsequent vision loss through retinal ganglion cell death. [2]
Signs and Symptoms
Glaucoma as a disease develops insidiously and may not present with vision deficits until considerable neural damage has occurred.[2][5] The primary chronic symptom of glaucoma is progressive vision loss; patients that develop loss of vision starting at the periphery describe this as “tunnel vision.” With respect to changes in visual acuity, patients have also reported light sensitivity and decreased ability to detect contrast, which is likely due to optic nerve damage.[6][7] Additionally, glaucoma patients also report glare, which may be due to glaucoma or medications used to treat glaucoma.[7] Acute symptoms include headache, eye pain, redness of the eye, excessive tearing, and sudden worsening of vision.[2][4][5]
Treatment
The treatment for glaucoma is diverse and involves increasing therapy until the IOP is decreased and the deterioration of the vision ceases. First-line treatment for chronic glaucoma is eye drops consisting of beta-blockers or a prostaglandin analog. Second line pharmacologic treatment involves using topical alpha-agonists and carbonic anhydrase inhibitors.[4][5] Oral medications can be added if the IOP remains uncontrolled and the patient is able to tolerate them.[4][5] In addition to medications, laser treatments, which can be done in the clinic, have been shown to successfully decrease IOP in eyes with glaucoma; popular laser treatment options include argon laser trabeculoplasty (ALT) and selective laser trabeculoplasty (SLT).[8][9] Surgery, in the form of trabeculectomy, which involves surgically creating a hole through the sclera to relieve intraocular pressure, or glaucoma drainage device implantation, can be considered if IOP control cannot be achieved through medications alone.[4][5][3][10] Given the successes of glaucoma drainage devices as presented in the Tube Versus Trabeculectomy Study, the popularity of tube shunt surgery as a surgical option has increased.[11]
Complications of Surgical Treatment
While surgical treatment for glaucoma is more definitive, it comes with a set of risks and complications that must be weighed against potential benefit. The more common complications of glaucoma surgery include infection, hyphema, hypotony, vision loss, bleb formation, suprachoroidal hemorrhage, diplopia, strabismus, and pupillary/iris abnormalities.[4][12] The objective of this article is to review pupillary abnormalities, specifically pupillary peaking and iris retraction, in greater detail and understand how often and how clearly they have been documented in the literature as a complication of glaucoma drainage device implantation surgery.
Pupillary Abnormalities after Glaucoma Drainage Device Implantation
Introduction
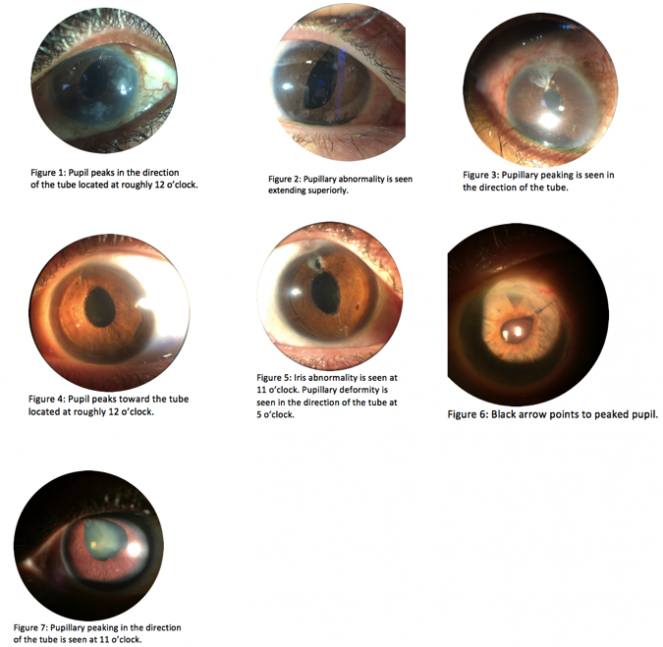
Pupillary peaking is a rare, but visually striking, complication of glaucoma tube-shunt surgery that entails retraction of the iris and asymmetric extension of the pupil in the direction of the tube.[13] Figures 1-7 provide examples of pupillary peaking seen after glaucoma tube shunt surgery; specifically, figures 1, 3, 4, 5, and 7 show pupillary peaking in the direction of tube’s opening. Previously, pupillary peaking has been documented as a complication of trauma and other eye surgeries including cataract extraction, intraocular lens (IOL) placement, and anterior vitrectomy.[14][15][16] Furthermore, it has also been documented in a lone case of pediatric mucogenic glaucoma prior to surgery.[17] Perhaps due to its rarity, pupillary peaking has not been well documented as a complication of glaucoma drainage device (GDD) implantation surgery in the literature.
Review of the Literature
Since the implementation and use of the Baerveldt and Ahmed Implants, large-scale studies have been done to evaluate the short and long-term complications of these surgeries. Though these studies have identified a myriad of post-operative and chronic complications, many do not clearly identify or describe pupillary peaking or distortion as a complication.[18][19][20][21][22][23] Of the few cases of post-tube-shunt surgery pupillary peaking that have been documented in the literature, most have been identified in pediatric eyes. The first documented case of a pupillary abnormality after GDD implantation was reported by Al-Torbak and Edwards in 2001, and occurred in a three-year-old girl with bilateral congenital glaucoma.[24] From their retrospective chart review of 60 pediatric eyes that had undergone Ahmed Valve placement, Morad et al. found that one eye had a complication in which the iris blocked the entrance of the tube, but there was no comment on peaking of the pupil.[11] In their six-case series on pediatric glaucoma, Pirouzian and Demer found that three eyes developed clinically insignificant but cosmetically prominent pupillary irregularities after Ahmed Glaucoma Valve placement.[13] Similarly, in their study of 79 eyes with congenital (38 eyes) and aphakic (41 eyes) glaucoma, O’Malley Schotthoeffer et al. found that 16% of eyes in the congenital glaucoma group and 7% of eyes in the aphakic glaucoma developed post-operative pupillary abnormalities.[25] Outside of these studies, pupillary peaking in the setting of GDD implantation surgery has not been well described.
Pathogenesis
The exact pathogenesis behind pupillary peaking after GDD implantation has not been clearly delineated, but researchers have put forward theories to attempt to explain this. Pirouzian and Demer surmised that the phenomenon of pupillary peaking may occur due to (1) pressure-flow turbulence that occurs at the tube and draws the iris toward the tube opening, (2) inflammation in response to talc on the tube, (3) formation of peripheral anterior synechia, (4) tube retraction and subsequent iris pull secondary to mechanical forces (i.e. eye rubbing), or (5) iris incarceration and entrapment in the setting of fistula creation.[13] Furthermore, they proposed two surgical techniques to prevent this complication: insertion of the tube posterior to the grey surgical limbus line and performing a surgical iridectomy at the entrance of the valve to alter fluid flow in that area.[13]
Effects on Visual Acuity
Just as there is a dearth of documentation of pupillary abnormalities after GDD implantation in the literature, there is also a paucity of information regarding the effects on visual acuity. In their case series, Pirouzian and Demer found that pupillary peaking was a visually insignificant complication.[13] Conversely, pupillary peaking in the setting of other ocular surgeries led to decreases in visual acuity. Masket reported a case of pupillary peaking contributing to glare in a post-operative patient.[26] Schechter documented a case of post-PCIOL placement pupillary peaking that led to a visual aberration; the visual aberration was eliminated after adhesions between the anterior capsule remnants and posterior iris surface were cleared.[27] Similarly, Khokhar et al. describe a case of pupillary peaking, secondary to persistent pupillary membrane, that led to a decrease in visual acuity to light perception in the affected eye.[14] Furthermore, because patients with glaucoma have underlying vision problems, it is difficult to evaluate the degree to which pupillary abnormalities contribute to vision loss. Ultimately, more research is needed to evaluate the impact of pupillary abnormalities on visual acuity in cases of glaucoma that undergo GDD implantation.
Treatment
The treatment of pupillary peaking depends on the cause. If the peaked pupil is the result of an iris or vitreous strand, the Nd:YAG laser can be used to break the strands that cause the traction; afterwards, pilocarpine eye drops may be used to bring the pupil back to its original shape.[28] Khokhar et al. similarly found that their case of pupillary peaking resolved with the use of pilocarpine and tropicamide eye drops.[14] In some cases, pupillary peaking may be a sign of ocular trauma; in these cases, the trauma to the eye and its sequelae must be addressed before the pupillary abnormalities.[29] Ultimately, the treatment for pupillary peaking depends on the etiology. Due to the lack of literature on pupillary peaking after GDD implantation, more research must be done to elucidate the pathophysiology so that the optimal treatment can be identified and employed.
References
- ↑ Tham, Yih-Chung, et al. “Global Prevalence of Glaucoma and Projections of Glaucoma Burden through 2040.” Ophthalmology, vol. 121, no. 11, 2014, pp. 2081–2090., doi:10.1016/j.ophtha.2014.05.013.
- ↑ Jump up to: 2.0 2.1 2.2 2.3 2.4 2.5 Weinreb, Robert N., et al. “The Pathophysiology and Treatment of Glaucoma.” JAMA, vol. 311, no. 18, 14 May 2014, pp. 1901–1911., doi:10.1001/jama.2014.3192.
- ↑ Jump up to: 3.0 3.1 3.2 3.3 Gupta, Divakar, and Philip P. Chen. “Glaucoma.” American Family Physician, 15 Apr. 2016, www.aafp.org/afp/2016/0415/p668.html.
- ↑ Jump up to: 4.0 4.1 4.2 4.3 4.4 4.5 See, Jovina LS, et al. “Management of Angle Closure Glaucoma.” Indian Journal of Ophthalmology, vol. 59, Jan. 2011, pp. S82–S87., doi:10.4103/0301-4738.73690.
- ↑ Jump up to: 5.0 5.1 5.2 5.3 5.4 Lee, David A., and Eve J. Higginbotham. “Glaucoma and Its Treatment: A Review.” American Journal of Health-System Pharmacy, vol. 62, no. 7, 1 Apr. 2005, pp. 691–699., doi:10.1093/ajhp/62.7.691.
- ↑ Hawkins, Anjali S, et al. “Comparison of Contrast Sensitivity, Visual Acuity, and Humphrey Visual Field Testing in Patients with Glaucoma.” Journal of Glaucoma, U.S. National Library of Medicine, Apr. 2003, www.ncbi.nlm.nih.gov/pubmed/12671468. Department of Ophthalmology and Visual Sciences, University of Illinois at Chicago College of Medicine, Chicago, Illinois 60612, USA.
- ↑ Jump up to: 7.0 7.1 Emanuel, Matthew. "Light Sensitivity and Glare with Glaucoma." Glaucoma Associates of Texas, 10 Dec. 2017, glaucomaassociates.com/glaucoma/Light-sensitivity-glare-glaucoma/.
- ↑ Koval, M. S., El Sayyad, F. F., Bell, N. P., Chuang, A. Z., Lee, D. A., Hypes, S. M., Grover, D. S., Baker, L. A., Huddleston, S. M., Budenz, D. L., Feldman, R. M. (2013). Risk factors for tube shunt exposure: a matched case-control study. Journal of ophthalmology, 2013, 196215.
- ↑ Sarkisian, Steven R. “Going With the Flow: Managing Tube Shunts.” Review of Ophthalmology, 18 Nov. 2009,
- ↑ Sarkisian SR, Netland PA. Tube extender for revision of glaucoma drainage implants. J Glaucoma. 2007;16:7:637-9.
- ↑ Jump up to: 11.0 11.1 Singh, K., & Gedde, S. J. (2011). Interpretation and misinterpretation of results from the tube versus trabeculectomy study. International ophthalmology clinics, 51(3), 141-54.
- ↑ Singh, K., & Gedde, S. J. (2011). Interpretation and misinterpretation of results from the tube versus trabeculectomy study. International ophthalmology clinics, 51(3), 141-54.
- ↑ Jump up to: 13.0 13.1 13.2 13.3 13.4 Pirouzian, Amir, and Joseph L Demer. “Clinical Findings Following Ahmed Glaucoma Valve™ Implantation in Pediatric Glaucoma.” Clinical Ophthalmology, vol. 2, no. 1, Mar. 2008, pp. 123–127., doi:10.2147/opth.s2025.
- ↑ Jump up to: 14.0 14.1 14.2 Khokhar, Sudarshan, et al. “Postoperative Pupillary Peaking in Case of Persistent Pupillary Membrane.” Journal of Cataract & Refractive Surgery, vol. 1, no. 1, June 2013, pp. e3-e5., doi:10.1016/j.jcro.2013.05.002
- ↑ Pandey, Sureshk, and Vidushi Sharma. “Commentary: Modified Sewing Machine Technique: An Innovative Method for the Management of Iridodialysis, Iris Coloboma, and Scleral Fixation of Intraocular Lenses.” Indian Journal of Ophthalmology, vol. 66, no. 8, Aug. 2018, pp. 1177–1178., doi:10.4103/ijo.ijo_731_18.
- ↑ Cubano, Miguel, editor. Emergency War Surgery. 5th ed., Borden Institute, U.S. Army Medical Department Center and School, 2013.
- ↑ Chang, Ta C., et al. “Mucogenic Glaucoma in a Child.” American Journal of Ophthalmology Case Reports, vol. 5, 26 Dec. 2016, pp. 85–89., doi:10.1016/j.ajoc.2016.12.011.
- ↑ Siegner, Scott W., et al. “Clinical Experience with the Baerveldt Glaucoma Drainage Implant.” Ophthalmology, vol. 102, no. 9, Sept. 1995, pp. 1298–1307., doi:10.1016/s0161-6420(95)30871-8.
- ↑ Souza, Carlos, et al. “Long-Term Outcomes of Ahmed Glaucoma Valve Implantation in Refractory Glaucomas.” American Journal of Ophthalmology, vol. 144, no. 6, Dec. 2007, pp. 893–900., doi:10.1016/j.ajo.2007.07.035.
- ↑ Ceballos, Elizenda M, et al. “Outcome of Baerveldt Glaucoma Drainage Implants for the Treatment of Uveitic Glaucoma.” Ophthalmology, vol. 109, no. 12, Dec. 2002, pp. 2256–2260., doi:10.1016/s0161-6420(02)01294-0.
- ↑ Riva, Ivano, et al. “Ahmed Glaucoma Valve Implant: Surgical Technique and Complications.” Clinical Ophthalmology, Volume 11, 17 Feb. 2017, pp. 357–367., doi:10.2147/opth.s104220.
- ↑ Nguyen, Quang H. “Complications of Baerveldt Glaucoma Drainage Implants.” Archives of Ophthalmology, vol. 116, no. 5, May 1998, pp. 571–575., doi:10.1001/archopht.116.5.571.
- ↑ Souza, Carlos, et al. “Long-Term Outcomes of Ahmed Glaucoma Valve Implantation in Refractory Glaucomas.” American Journal of Ophthalmology, vol. 144, no. 6, Dec. 2007, pp. 893–900., doi:10.1016/j.ajo.2007.07.035.
- ↑ Al-Torbak, Abdullah. “Transcorneal Tube Erosion of an Ahmed Valve Implant in a Child.” Archives of Ophthalmology, vol. 119, no. 10, Oct. 2001, pp. 1558–1559., doi:10.1001/archopht.119.10.1558.
- ↑ O’Malley Schotthoefer, Erin, et al. “Aqueous Drainage Device Surgery in Refractory Pediatric Glaucomas: I. Long-Term Outcomes.” Journal of American Association for Pediatric Ophthalmology and Strabismus, vol. 12, no. 1, 12 Feb. 2008, pp. 33–39., doi:10.1016/j.jaapos.2007.07.002.
- ↑ Masket, Samuel. “Peaked Pupil One Day After Cataract Surgery.” Journal of Cataract & Refractive Surgery, vol. 41, no. 8, 2015, p. 1787., doi:10.1016/j.jcrs.2015.07.021.
- ↑ Schechter, Robert J. “Pupillary Peaking with Exposure of an Intraocular Lens Positioning Hole Corrected by Nd:YAG Laser Treatment.” Journal of Cataract & Refractive Surgery, vol. 14, no. 1, Jan. 1988, pp. 86–87., doi:10.1016/s0886-3350(88)80072-5.
- ↑ Bellucci, Roberto, et al. “Peaked Pupil.” CRSTEurope, Jan. 2007, crstodayeurope.com/articles/2007-jan/0107_08-php/.
- ↑ Pramanik, Sudeep, et al. “Assessment and Management of Ocular Trauma.” Ocular Trauma, Assessment and Management, 28 Jan. 2008, webeye.ophth.uiowa.edu/eyeforum/tutorials/trauma.htm.