Punctate Inner Choroidopathy
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
Punctate inner choroidopathy (PIC) is an inflammatory disease affecting the choroid and retina, which can lead to vision loss, often in young women.
Disease Entity
- ICD9: 363.20 chorioretinitis, unspecified
- ICD10: H30.9 Chorioretinal inflammation, unspecified
- ICD10: H30.1 Disseminated chorioretinal inflammation
Disease
Punctate inner choroidopathy (PIC) is an idiopathic inflammatory disorder of the choroid which was first described by Watzke et al in 1984[1].
Other names: Punctate inner choroiditis, multifocal inner choroiditis[2]
Etiology
The etiology has remained unclear, and it is proposed to be an autoimmune disease that arises in the context of polygenic susceptibility triggered by an environmental stimulus, such as infection, immunization, or stress.[2]
PIC was proposed to be a variant of multifocal choroiditis and panuveitis (MFCPU), a form of limited myopic degeneration or a variant of Multifocal Choroiditis (MFC).[3]
A previous study suggested an association between MFC and Epstein-Barr (EB) virus infection, because patients with MFC had higher EB antibody titers for the early antigens[4]. Incidence and reactivation of PIC following COVID-19 infection have also been reported. Sars-CoV-2 infection induces autoimmune and autoinflammatory dysregulation in genetically predisposed subjects.[5][6]
Recent reports have also reported haplotype associations between MFCPU and PIC, given their similar genetic associations with IL10 and TNF loci[7].
Other studies have reported an association between PIC and HLA -DR2 and indeed, there have been reports of familial cases, such as in a mother-daughter cohort[8] [9].
Risk Factors
It predominantly occurs in myopic females (90%), usually aged 18 to 40 years of age although more recent studies have reported a slightly different spectrum with the mean age of presentation in one case series reported to be 32 years with a range of 24 to 52 years[10].
In the original case series by Watzke et al, myopia ranged from -3.25 to - 10.0 Diopters[1]. Reddy and colleagues noted that patients with PIC had the highest level of myopia of all inflammatory chorioretinopathies.[11]
General Pathology
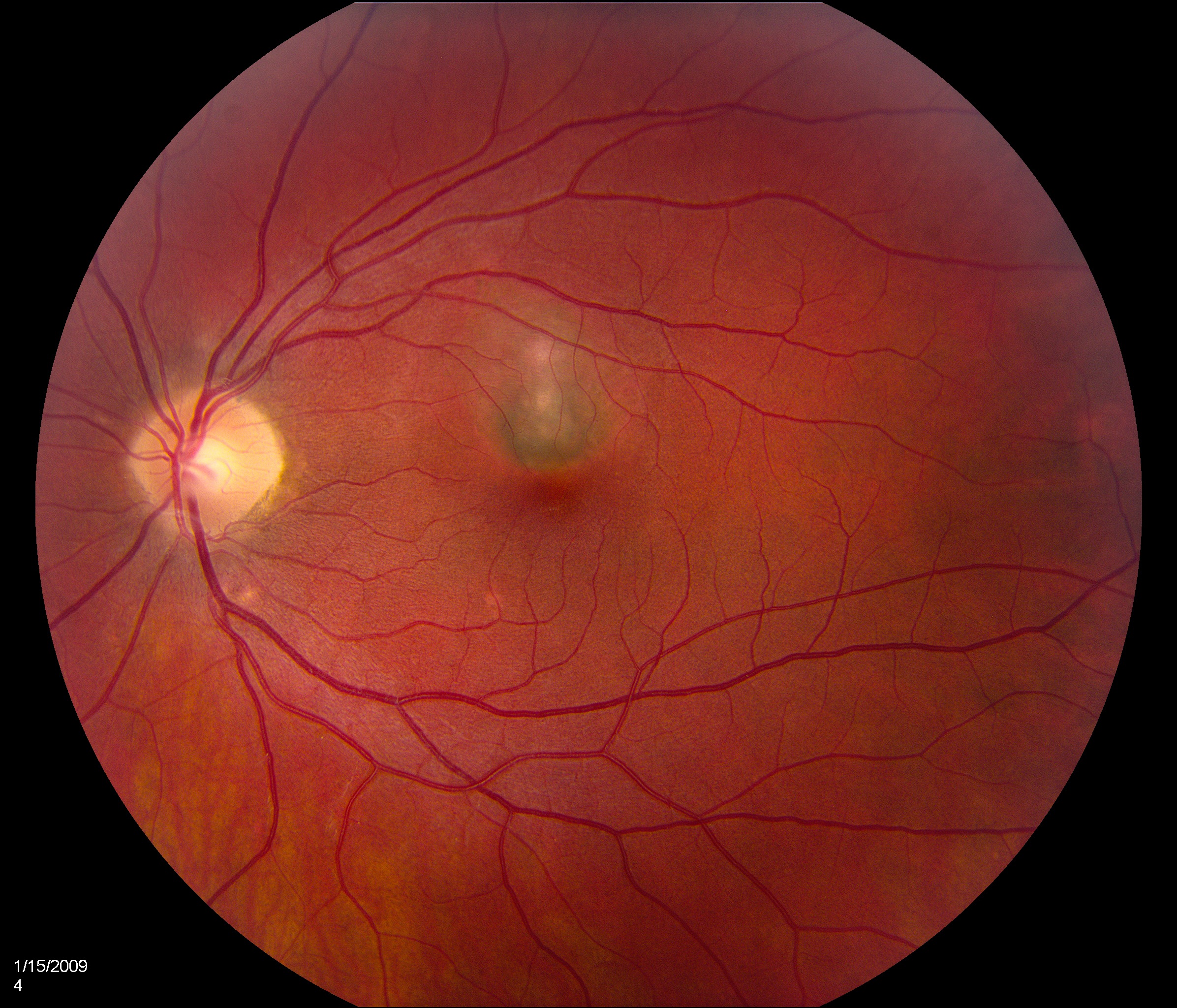
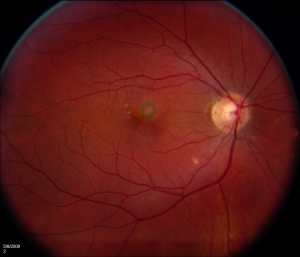
Bilateral white-yellow chorioretinal lesions usually 100-200 microns diameter develop at the level of the inner choroid and retinal pigment epithelium (RPE) which rarely extend to the midperiphery and are never associated with vitritis. They are bilateral in 80% of cases but are usually asymmetric. They progress to atrophic scars, leaving a halo of depigmentation and are deeper and appear punched-out. Subretinal neovascular membranes in this condition occur in between 40 to 75% of cases depending on case examined[10].
Histopathology
A recent pathological study examining choroidal neovascular membranes (CNVMs) secondary to PIC showed some intriguing findings[12]. Light and electron microscopy of the CNVM showed lymphocytes at the level of the inner choroid with sparing of the choriocapillaris. This study provided ultrastructural electron microscopic support to the hypothesis that PIC is an inflammatory disease, with the inflammation originating in the choroid. Pericyte-poor neovascular units have been shown to be more susceptible to one type of treatment (anti-VEGF agents) than pericyte-rich ones. This is the first pathological study employing human tissue that points to pericytes as a potential critical therapeutic target with the aggravating influence of inner choroidal chronic inflammation in PIC[12].
Pathophysiology
There are several theories as to the etiology including those discussed above (eg an inflammatory or infectious thrombosis of the choriocapillaris layer by an unidentified organism), however the specific mechanism remains elusive.
Primary Prevention
There are no known preventive measures for PIC.
Diagnosis
Diagnosis is based on clinical examination. Ancillary tests from the options below can be used as adjuncts in difficult cases.
History
Patients usually complain of blurred vision, scotoma, and floaters at presentation.
Physical Examination
Evaluation of patients with suspected punctate inner choroidopathy includes a complete ophthalmological exam.
Symptoms
Blurred vision, photopsia, central and/or peripheral scotomatas and metamorphopsias. Studies have reported the commonest reported initial symptoms are unilateral scotoma and blurred vision[13].
Signs
The initial visual acuity at presentation varies from 20/50 to 20/400. In the original report by Watzke et al, 8 of 12 eyes had VA of 20/50 or better (66.7%), 2 had 20/70, one had 20/500, and another had counting fingers[1].
Brown et al reported that 88% of patients with PIC had bilateral disease, compared to 66% in MCP, 100% in DSF (diffuse subretinal fibrosis), and 25% in MEWDS patients[14].
Patients tend to be myopic. Fundoscopic features of PIC include small (100-300 microns), well-defined, yellow-gray spots (12-25 in number) normally limited to the posterior pole and distributed in a random (or rarely, linear) pattern. These inflammatory lesions occur at the outer retina, RPE, and inner choroid levels and may be associated with an overlying neurosensory detachment. They spare the peripapillary region. Typically, PIC is not associated with signs of intraocular inflammation elsewhere in the eye.[2]
Clinical Diagnosis
Diagnosis is based on history and physical.
Natural history
The inflammatory PIC lesions usually resolve within a few weeks with full symptomatic visual recovery despite cause permanent structural changes that may be detected on multimodal imaging.[2]
Some patients develop atrophic chorioretinal scars at the sites of previous inflammation, which may become increasingly well-defined (“punched out”) and pigmented. Even without any ongoing inflammation, these scars may gradually increase in size, leading to worsening symptoms over time, especially if they arise in lesions abutting the fovea.[2]
Diagnostic procedures
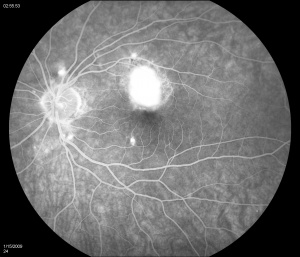
Fluorescein angiography
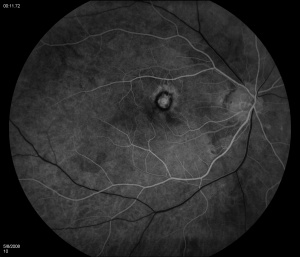
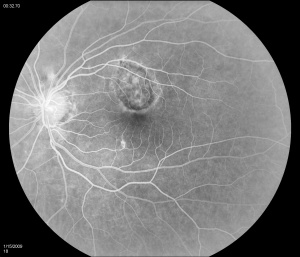
Fluorescein angiography (FA) shows early hyperfluorescence, variable late leakage/staining of acute lesions, leakage in presence of cystoid macular edema (CME) and choroidal neovascular membrane (CNVM). PIC lesions are hyperfluorescent in the early arterial phase, with staining observed in the arteriovenous phase. In some cases the lesions blocked fluorescence in the early arterial phase and stained thereafter. More lesions were seen on FA than on clinical examination. As the disease progresses damage to the RPE occurs and FA demonstrates punctate RPE window defects. Leakage of fluorescein into the subretinal space was observed in patients with a serous neurosensory retinal detachment[1]. Descriptions of both the pathology and clinical features of CNVMs in PIC have also been reported. Olsen et al described the FA characteristics in 6 eyes. PIC CNVMs appeared as focal areas with an irregular, lacy network of neovascularization, with hyperfluorescence in the early phase and leakage in the late phase. Over time the newer vessels linked to form a larger neovascular complex with multiple feeder vessels originating from individual neovascular buds. The subsequent fibrotic response leads to a dumbbell-shaped subretinal fibrosis pattern [15].
Indocyanine green (ICG)
Indocyanine green (ICG) shows multiple midphase hypofluorescent lesions in the peripapillary posterior pole, corresponding to those seen on exams. ICG is a useful tool in the diagnosis of PIC. It has been reported to show subclinical hypofluorescent spots in 32% of affected eyes, thereby increasing the diagnostic potential in patients who have evaded clinical diagnosis[16]. Tiffin et al described unusual abnormalities of the choroidal vasculature in PIC[17]. Several areas of obvious hypofluorescence corresponded to the site of the visible subretinal lesions; larger choroidal vessels were noted to cross these areas. In addition, several choroidal vessels demonstrated localized points of hyperfluorescence situated close to the vessel wall/border. The authors suggested that the hypofluorescent areas corresponded to localized choroidal hypoperfusion, whereas the localized points of hyperfluorescence on the vessel walls might indicate an associated vasculitis. The presence of larger choroidal vessels running through the hypofluorescent areas could imply that the vasculitic process is confined to smaller choroidal vessels and the choriocapillaris[17].
Visual fields
Visual fields show enlargement of the blind spot in approximately 41% of cases and central and paracentral scotoma. Watzke cited the occurrence of relative scotomata at the onset of the disease, although no details were given with regards to the type of visual field (VF) defect present or their course over time[1]. In a report on 25 patients who presented with enlarged blind spots, 17 (68%) had clinical findings compatible with a concomitant chorioretinal disorder, including MEWDS, PIC, MCP, and acute macular neuroretinopathy[18]. Other studies showed 45% of patients had normal visual fields[19].This study showed the most frequent VF defect detected was enlargement of the blind spot in 41% of eyes (nine eyes). Central/paracentral scotomata were detected in 14% (three eyes). No cecocentral or peripheral scotomata were observed. In many patients the blind spot extended towards the macula and the authors theorized this may have been due to the peripapillary clustering of the inflammatory lesions[20]. Followup of this same group of patients revealed an improvement in most visual fields without treatment which was in contrast to patients with acute idiopathic blind spot enlargement syndrome.
OCT
Spectral domain-ocular coherence tomography (SD-OCT) has been reported to be a useful tool in the diagnostic armamentarium and for following conditions affecting outer retinal structures.
Optical coherence tomography (OCT) demonstrates focal hyperreflective elevation of the retinal pigment epithelium with corresponding inner and outer segment photoreceptor interface disruption.[21]
Spectral-domain OCT characterized a 5-stage evolution of PIC lesions: choroidal infiltration, formation of sub-RPE nodules, and then chorioretinal nodules, regression, and retinal herniation.[22]
Quantitative and qualitative OCT analysis can aid in predicting an underlying CNV in the eyes with PIC. Lesions with CNV+ had larger height, width, and volume, disruption of EZ and BM, outer retinal fuzziness, and hypo-reflective back-shadowing compared with CNV-lesions.[23] [24]
Enhanced depth imaging OCT of acute lesions may demonstrate increased choroidal thickening.[25]EDI-OCT can also help differentiate inflammatory CNV from myopic CNV in PIC. Choroidal thickness beneath inflammatory choroidal neovascularization significantly increased at baseline and decreased after therapy (“Sponge sign”), reaching preclinical values. Conversely, no significant choroidal thickness changes were disclosed in myopic choroidal neovascularization eyes, under any location.[26]
OCTA showed the active inflammatory chorioretinal lesions as non-detectable flow signals in choriocapillaris and choroid.[27]OCTA may be helpful in the detection, follow-up, and evaluation of therapeutic strategies to treat CNV secondary to PIC.[28]The use of OCT angiography (OCTA) on PIC lesions may enable differentiation between active inflammatory lesions and CNV. Levison and colleagues conducted OCTA in 12 patients with PIC and noted that OCTA was able to demonstrate the presence of a CNV in 11 patients, including all those in whom FFA had been inconclusive,[29]
Fundus autofluorescence
In a study by Turkcuoglu et al [30], active PIC lesions were noted to a hyperautofluorescent halo surrounding the active lesion and that a hyperautofluorescence halo may be an indirect sign of uncontrolled inflammation. In their case series, patients that had a clinical response to immunomodulatory therapy an associated decrement in the hyperautofluorescence halo was also noted.
Heatmap Analysis
A heatmap analysis of choroidal lesions in patients with punctate inner choroidopathy (PIC) or multifocal choroiditis (MFC) with or without uveitis was performed to determine if there were any distinguishing features among these uveitic entities.
Heatmap analysis revealed three distinct patterns of fundus lesions: posterior, peripheral, and combined. All patients with PIC had the posterior pattern. Patients with MFC had the peripheral or combined pattern, and all patients with MFC with uveitis had the combined pattern.[31]
Electrophysiology
Electroretinogram (ERG) is typically normal. In one electrophysiological study 7 out of 16 patients with PIC demonstrated a normal full-field electroretinogram. Three of the seven patients (42.8%) had mild asymmetry in b-wave amplitudes between the two involved eyes that correlated with differences in the number of chorioretinal lesions present in each eye[20]. Electrooculogram (EOG) can demonstrate very mild abnormalities of the Arden ratio due to involvement of the retinal pigment epithelial layer.Laboratory test
The diagnosis of PIC is largely based on clinical findings. Adjunctive testing such as FA and ICG mentioned above are also helpful particularly in less typical or early forms. Histoplasmosis skin testing is negative.
Differential diagnosis
Differential diagnosis includes Acute Posterior Multifocal Plaquoid Pigment Epitheliolopathy, Behcets’ disease, Harada disease, Leukemia, Myopic degeneration, Multiple evanescent white dot syndrome (MWEDS), Pars planitis, Presumed ocular histoplasmosis, Sarcoidosis, Sympathetic ophthalmia, Serpiginous choroiditis, Vogt-Koyanagi-Harada disease or Whipples disease.
Management
General treatment
No treatment is advised for the majority of patients with PIC when there is no evidence of CNV as the visual prognosis is excellent. The only exception to this would be those patients with inflammatory lesions very close to fixation in whom medical treatment may be considered. Additionally patients who have developed CNVMs should also be considered for treatment as discussed below.
Medical therapy
Systemic corticosteroids
Systemic corticosteroids have been used alone or combined as part of a multimodal approach. The usual starting dose is 1 mg/kg (60- 80 mg oral daily) for 3-5 days and subsequently tapered[32]. Lesions may show a marked improvement however this may be without an improvement in visual acuity due to CNVM formation and subsequent subfoveal fibrosis[33].
One case report showed the value of oral steroids in a 28 year old pregnant female with PIC after intravitreal lucentis and PDT have failed to arrest disease progression[34]. Interestingly one would expect that inflammatory activity of PIC or other autoimmune inflammatory diseases would be suppressed during pregnancy and exacerbated in the postpartum period[35][36]. A case report by Rao et al demonstrated a flare up of choroiditis in the first trimester[34]
The multimodal approach to treatment has also been used in the management of PIC. One such study examined 5 patients treated with PDT combined with oral prednisolone (1 mg/kg body weight/day) which was started 5 days before PDT over a 12 month followup period and found a mean improvement in vision of 15 letter following a mean of 2 PDT treatments[37].
Mycophenolate mofetil
Mycophenolate mofetil suppresses the immune system by selectively inhibiting the purine biosynthesis enzyme inosine monophosphate dehydrogenase (IMPDH), thereby resulting in the depletion of guanosine nucleotides that are essential for purine synthesis used in the proliferation of B and T lymphocytes[38]. Mycophenolate mofetil reduces the frequency of attacks in recurrent PIC. This was used in conjunction with fundus autofluorescence to monitor and predict the response to treatment[39].
Sirolimus (rapamycin)
Sirolimus inhibits the secretion of IL-2 and has been reported to be used successfully in a patient with juxtafoveal PIC-associated CNVM[40].
Interferon B-1A
One study reported the resolution of disease activity following the treatment of chronic recurrent PIC with interferon B-1A[41]. There have been scant reports on this specific modality of treatment for PIC in the literature.
Intraocular corticosteroid implants and injections
Intravitreal triamcinolone
One of the more commonly used methods of administration has been the intravitreal injection of 4 mg of triamcinolone. One recent retrospective study studied fourteen patients (14 eyes) over 12 month follow-up who had PIC and idiopathic CNVM. Patients were treated with combined intravitreal triamcinolone (4 mg) and PDT. The mean logMAR BCVA improved significantly from 0.52 at baseline to 0.20 at 1 year (Wilcoxon signed- ranks test, P = 0.003)[42].
Intravitreal dexamethasone implant
More recently an intravitreal implant containing 0.7 mg or 0.35 mg of dexamethasone for posterior uveitis releases the medication over a 6 month period. It has been used along with intravitreal anti-VEGF agents to treat PIC complicated by CNVM.[43] [44]
Intravitreal fluocinolone acetonide implants
Injectable, non-biodegradable, intravitreal implants containing 0.59 mg of fluocinolone acetonide releases its contents over 36 months. The medication is released at a nominal initial rate of 0.6 μg/day, decreasing over the first month to a steady state between 0.3-0.4 μg/day over approximately 30 months.
Local therapy may be preferred, particularly in women planning pregnancy and if intolerant to systemic therapy. Long-acting intravitreal steroid achieved stability in these patients and permitted a reduction of systemic treatment burden.[45]
Intravitreal bevacizumab and ranibizumab
Several case series have reported the successful treatment of CNVM with anti-VEGF treatments[46] [47]. Although anti-VEGF agents have not been examined in pregnant patients with PIC, it has been successfully used in the treatment of CNVM with good results[48]. Rouvas et al followed a cohort of 16 patients including 5 with PIC over a period of 70 weeks following intravitreal injection of ranibizumab[49]. They found an improvement in mean foveal thickness and visual acuity as well as significant regression in CNVM over the course of the study[49]. It remains to be seen whether the advent of VEGF-TRAP holds the key to widening the anti-VEGF spectrum for the white dot syndromes, including PIC. Without treatment CNV is inevitably progressive[50] [51].
Photodynamic therapy
Several reports have substantiated PDT as an effective treatment option in extrafoveal or juxtafoveal CNV due to PIC. PDT has been advocated as a viable option if outcome without treatment is likely to be poor, and preliminary success in ocular histoplasmosis syndrome, angioid streaks, idiopathic, and other conditions has been reported[52] [53] [54] [55] [56] [57] [58] [59]. With the widespread use of anti-VEGF treatment its role continues to decline. Studies of subfoveal CNVs which had failed to improve with a single dose of immunosuppressive therapy showed an improvement in visual acuity after they were treated with PDT[60]. A multimodal approach using a combination of PDT and intravitreal triamcinolone have also been used for the treatment of CNV[61]. This was described in a cohort of 15 patients who showed a significant improvement in visual acuity at 3 and 6 months but a worsening at 12 months[61]. Although PDT can be useful in selective circumstances, its role remains limited in CNV secondary to PIC.
Medical follow up
Patients are followed at periodic intervals by a uveitis/retinal specialist depending on level of inflammation/pathology.
Surgery
Submacular translocation surgery
Although currently submacular translocation surgery is no longer advocated for ARMD related CNVM, recent studies have examined its use in a cohort of patients with progressive use from non-ARMD submacular diseases including PIC. They primarily examined final visual acuity and found a large percentage of subjects gained >3 lines of visual acuity (38%) and achieved a final visual acuity of ≥ 20/50 (31%) over a mean followup of 28 months[62]. The submacular surgery trial examined a cohort of patients following submacular surgery and recurrent CNV developed in 58 % of patients. One recent publication examined the ultrastructural and pathological features of CNVMs in PIC in a patient with PIC who initially had intravitreal bevacizumab followed by submacular surgery when this failed[63]. This study noted recurred in on eye of a PIC patient with bilateral CNVMs who had submacular surgery in both eyes. This was consistent with the study by Olsen et al in which four out of six eyes developed a recurrence of CNV following surgical excision[15].
Surgical follow up
Close follow up after surgical intervention is necessary. Patients should be monitored for recurrence of disease.
Complications
CNVM as well as subretinal fibrosis can develop leading to poorer visual outcomes.
Over time, certain patterns of fibrosis may be seen including peripapillary (“napkin ring” appearance) and “bridging” fibrosis between scars.[64]
The Gerstenblith survey reported that around one-third of those who developed CNV or subretinal fibrosis in 1 eye developed the same complication in the other.[13]
Prognosis
Visual prognosis is good in the absence of CNVM with 50-75% of eyes having visual acuity better than 20/25. The course is usually self-limited with recurrences common, usually in the first 3 months. [65] The two major causes of visual loss are CNVM and subretinal fibrosis. One study of 136 patients noted CNVM in 74 (66%) of cases. In eyes with choroidal neovascularization, the mean logMAR visual acuity was 0.63 at study entry, 0.63 at 12 months, 0.61 at 2 years, and 0.71 at final review (mean, 6.1 years). Brown et al reported a cohort with a mean length of followup of 51 months. The final average VA was 20/40 or better in 77% of eyes (23 eyes) and 20/50 or worse in 23% (7 eyes). In 20% of eyes (6 eyes) it was 20/200 or worse[14].
References
- ↑ Jump up to: 1.0 1.1 1.2 1.3 1.4 Watzke RC, Packer AJ, Folk JC, Benson WE, Burgess D, Ober RR. Punctate inner choroidopathy. Am J Ophthalmol. 1984 Nov;98(5):572-84.
- ↑ Jump up to: 2.0 2.1 2.2 2.3 2.4 Ahnood D, Madhusudhan S, Tsaloumas MD, Waheed NK, Keane PA, Denniston AK. Punctate inner choroidopathy: A review. Surv Ophthalmol. 2017 Mar-Apr;62(2):113-126. doi: 10.1016/j.survophthal.2016.10.003. Epub 2016 Oct 15. PMID: 27751823.
- ↑ Jampol LM, Becker KG. White spot syndromes of the retina: a hypothesis based on the common genetic hypothesis of autoimmune/inflammatory disease. Am J Ophthalmol. 2003 Mar;135(3):376-9. doi: 10.1016/s0002-9394(02)02088-3. PMID: 12614757.
- ↑ Tiedman JS. Epstein-Barr viral antibodies in multifocal choroiditis and panuveitis. Am J Ophthalmol 1987;103:659–663.
- ↑ Miyata M, Ooto S, Muraoka Y. Punctate inner choroidopathy immediately after COVID-19 infection: a case report. BMC Ophthalmol. 2022 Jul 7;22(1):297. doi: 10.1186/s12886-022-02514-8. PMID: 35799141; PMCID: PMC9260973.
- ↑ Nicolai M, Carpenè MJ, Lassandro NV, Pelliccioni P, Pirani V, Franceschi A, Mariotti C. Punctate inner choroidopathy reactivation following COVID-19: A case report. Eur J Ophthalmol. 2022 Jul;32(4):NP6-NP10. doi: 10.1177/11206721211028750. Epub 2021 Jul 3. PMID: 34219492; PMCID: PMC9294619.
- ↑ Atan D, Fraser-Bell S, Plskova J, Kuffová L, Hogan A, Tufail A, Kilmartin DJ, Forrester JV, Bidwell JL, Dick AD, Churchill AJ. Punctate inner choroidopathy and multifocal choroiditis with panuveitis share haplotypic associations with IL10 and TNF loci. Invest Ophthalmol Vis Sci. 2011 Jun 1;52(6):3573-81.
- ↑ Spaide RF, Skerry JE, Yannuzzi LA, DeRosa JT. Lack of the HLA-DR2 specificity in multifocal choroiditis and panuveitis. Br J Ophthalmol 1990;74:536–537.
- ↑ Sugawara E, Machida S, Fujiwara T, Kurosaka D, Hayakawa M. Punctate inner choroidopathy in mother and daughter. Jpn J Ophthalmol. 2010 Sep;54(5):505-7.
- ↑ Jump up to: 10.0 10.1 Patel KH, Birnbaum AD, Tessler HH, Goldstein DA. Presentation and outcome of patients with punctate inner choroidopathy at a tertiary referral center. Retina. 2011 Jul-Aug;31(7):1387-91.
- ↑ Reddy CV, Brown J Jr, Folk JC, Kimura AE, Gupta S, Walker J. Enlarged blind spots in chorioretinal inflammatory disorders. Ophthalmology. 1996 Apr;103(4):606-17. doi: 10.1016/s0161-6420(96)30645-3. PMID: 8618760.
- ↑ Jump up to: 12.0 12.1 Pachydaki SI, Jakobiec FA, Bhat P, Sobrin L, Michaud NA, Seshan SV, D'Amico DJ. Surgical management and ultrastructural study of choroidal neovascularization in punctate inner choroidopathy after bevacizumab. J Ophthalmic Inflamm Infect. 2011 Nov 27.
- ↑ Jump up to: 13.0 13.1 Gerstenblith AT, Thorne JE, Sobrin L, Do DV, Shah SM, Foster CS, Jabs DA, Nguyen QD. Punctate inner choroidopathy: a survey analysis of 77 persons. Ophthalmology. 2007 Jun;114(6):1201-4.
- ↑ Jump up to: 14.0 14.1 Brown J Jr, Folk JC, Reddy CV, et al. Visual prognosis of multifocal choroiditis, punctate inner choroidopathy, and the diffuse subretinal fibrosis syndrome. Ophthalmology. 1996;103:1100--5
- ↑ Jump up to: 15.0 15.1 Olsen TW, Capone A, Sternberg P, et al. Subfovealchoroidal neovascularization in punctate inner choroidopathy. Surgical management and pathologic findings. Ophthalmology. 1996;103:2061--9
- ↑ Zhang X, Wen F, Zuo C, Li M, Chen H, Huang S, Luo G. Clinical features of punctate inner choroidopathy in Chinese patients. Retina. 2011 Sep;31(8):1680-91.
- ↑ Jump up to: 17.0 17.1 Tiffin PA, Maini R, Roxburgh ST, et al. Indocyanine green angiography in a case of punctate inner choroidopathy. Br J Ophthalmol. 2002;80:90--1
- ↑ Watzke RC, Shults WT. Clinical features and natural history of the acute idiopathic enlarged blind spot syndrome. Ophthalmology. 2002;109:1326--35
- ↑ Stepien KE, Carroll J. Using spectral-domain optical coherence tomography to follow outer retinal structure changes in a patient with recurrent punctate inner choroidopathy. J Ophthalmol. 2011;2011:753741.
- ↑ Jump up to: 20.0 20.1 Reddy CV, Brown J, Folk JC, et al. Enlarged blind spots in chorioretinal inflammatory disorders. Ophthalmology. 1996;103:606--17
- ↑ Jo Y, Gomi F, Ikuno Y. Spectral-domain optical coherence tomographic findings in punctate inner choroidopathy. Retin Cases Brief Rep. 2012 Spring;6(2):189-92. doi: 10.1097/ICB.0b013e31822476ea. PMID: 25390960.
- ↑ Zhang X, Zuo C, Li M, Chen H, Huang S, Wen F. Spectral-domain optical coherence tomographic findings at each stage of punctate inner choroidopathy. Ophthalmology. 2013 Dec;120(12):2678-2683. doi: 10.1016/j.ophtha.2013.05.012. Epub 2013 Jun 12. PMID: 23769333.
- ↑ Chen Y, Chen Q, Li X, Li M. RPE disruption and hyper-transmission are early signs of secondary CNV with punctate inner choroidopathy in structure-OCT. BMC Ophthalmol. 2021 Dec 10;21(1):427. doi: 10.1186/s12886-021-02197-7. PMID: 34893049; PMCID: PMC8662850.
- ↑ Agarwal A, Handa S, Marchese A, Parrulli S, Invernizzi A, Erckens RJ, Berendschot TTJM, Webers CAB, Bansal R, Gupta V. Optical Coherence Tomography Findings of Underlying Choroidal Neovascularization in Punctate Inner Choroidopathy. Front Med (Lausanne). 2021 Dec 22;8:758370. doi: 10.3389/fmed.2021.758370. PMID: 35004727; PMCID: PMC8727437.
- ↑ Zarranz-Ventura J, Sim DA, Keane PA, Patel PJ, Westcott MC, Lee RW, Tufail A, Pavesio CE. Characterization of punctate inner choroidopathy using enhanced depth imaging optical coherence tomography. Ophthalmology. 2014 Sep;121(9):1790-7. doi: 10.1016/j.ophtha.2014.03.011. Epub 2014 May 20. PMID: 24856311.
- ↑ Giuffrè C, Marchese A, Fogliato G, et al. The “Sponge sign”: A novel feature of inflammatory choroidal neovascularization. European Journal of Ophthalmology. 2021;31(3):1240-1247. doi:10.1177/1120672120917621
- ↑ Melachuri S, Dansingani KK, Wesalo J, Paez-Escamilla M, Gagrani M, Atta S, Indermill C, Sahel JA, Nischal KK, Chhablani J, Errera MH. OCT Angiography in Noninfectious Uveitis: A Description of Five Cases and Clinical Applications. Diagnostics (Basel). 2023 Mar 30;13(7):1296. doi: 10.3390/diagnostics13071296. PMID: 37046514; PMCID: PMC10092962.
- ↑ Nakao S, Kaizu Y, Oshima Y, Sakamoto T, Ishibashi T, Sonoda KH. Optical Coherence Tomography Angiography for Detecting Choroidal Neovascularization Secondary to Punctate Inner Choroidopathy. Ophthalmic Surg Lasers Imaging Retina. 2016 Dec 1;47(12):1157-1161. doi: 10.3928/23258160-20161130-13. PMID: 27977842.
- ↑ Levison AL, Baynes KM, Lowder CY, Kaiser PK, Srivastava SK. Choroidal neovascularisation on optical coherence tomography angiography in punctate inner choroidopathy and multifocal choroiditis. Br J Ophthalmol. 2017 May;101(5):616-622. doi: 10.1136/bjophthalmol-2016-308806. Epub 2016 Aug 18. PMID: 27539089.
- ↑ Turkcuoglu P, Chang PY, Rentiya ZS, Channa R, Ibrahim M, Hatef E, Sophie R, Sadaka A, Wang J, Sepah YJ, Do DV, Foster CS, Nguyen QD. Mycophenolate mofetil and fundus autofluorescence in the management of recurrent punctate inner choroidopathy. Ocul Immunol Inflamm. 2011 Aug;19(4):286-92.
- ↑ Park JG, Halim MS, Uludag G, Onghanseng N, Sredar N, Sepah YJ, Nguyen QD. Distinct Patterns of Choroidal Lesions in Punctate Inner Choroidopathy and Multifocal Choroiditis Determined by Heatmap Analysis. Ocul Immunol Inflamm. 2022 Feb 17;30(2):276-281. doi: 10.1080/09273948.2021.1939391. Epub 2021 Jul 6. PMID: 34228580.
- ↑ Levy J, Shneck M, Klemperer I, Lifshitz T. Punctate inner choroidopathy: resolution after oral steroid treatment and review of the literature. Can J Ophthalmol. Oct 2005;40(5):605-8.
- ↑ Brueggeman RM, Noffke AS, Jampol LM. Resolution of punctate inner choroidopathy lesions with oral prednisone therapy. Arch Ophthalmol. 2002 Jul;120(7):996.
- ↑ Jump up to: 34.0 34.1 Rao VG, Rao GS, Narkhede NS Flare up of choroiditis and choroidal neovasculazation associated with punctate inner choroidopathy during early pregnancy. Indian J Ophthalmol. 2011 Mar-Apr;59(2):145-8.
- ↑ Schultz KL, Birnbaum AD, Goldstein DA. Ocular disease in pregnancy. Curr Opin Ophthalmol. 2005;16:308–14
- ↑ Kump LI, Androudi SN, Foster CS. Ocular toxoplasmosis in pregnancy. Clin Experiment Ophthalmol. 2005 Oct;33(5):455-60.
- ↑ Fong et al 2008 Fong KC, Thomas D, Amin K, Inzerillo D, Horgan SE. Photodynamic therapy combined with systemic corticosteroids for choroidal neovascularisation secondary to punctate inner choroidopathy. Eye (Lond). 2008 Apr;22(4):528-33.
- ↑ Allison, AC. Mechanisms of action of mycophenolate mofetil. Lupus, 14 (2005), pp. S2 S8.
- ↑ Turkcuoglu P, Chang PY, Rentiya ZS, Channa R, Ibrahim M, Hatef E, Sophie R, Sadaka A, Wang J, Sepah YJ, Do DV, Foster CS, Nguyen QD. Mycophenolate mofetil and fundus autofluorescence in the management of recurrent punctate inner choroidopathy. Ocul Immunol Inflamm. 2011 Aug;19(4):286-92.
- ↑ Nussenblatt RB, Coleman H, Jirawuthiworavong G, et al. The treatment of multifocal choroiditis associated choroidal neovascularization with sirolimus (rapamycin). Acta Ophthalmol Scand. 2007;85:230-1
- ↑ Cirino AC, Mathura JR Jr, Jampol LM. Resolution of activity (choroiditis and choroidal neovascularization) of chronic recurrent punctate inner choroidopathy after treatment with interferon B-1A. Retina. 2006 Nov-Dec;26(9):1091-2.
- ↑ Chan WM, Lai TY, Lau TT, Lee VY, Liu DT, Lam DS. Combined photodynamic therapy and intravitreal triamcinolone for choroidal neovascularization secondary to punctate inner choroidopathy or of idiopathic origin: one-year results of a prospective series. Retina. 2008 Jan;28(1):71-80.
- ↑ Tsaousis KT, Nassr M, Kapoor B, Konidaris VE, Tyradellis S, Empeslidis T. Long-term results of intravitreal bevacizumab and dexamethasone for the treatment of punctate inner choroidopathy associated with choroidal neovascularization: A case series. SAGE Open Med Case Rep. 2018 May 6;6:2050313X18772478. doi: 10.1177/2050313X18772478. PMID: 29760922; PMCID: PMC5946604.
- ↑ Wu W, Li S, Xu H, Liu Y, Wang Y, Lai TYY, Yin ZQ. Treatment of Punctate Inner Choroidopathy with Choroidal Neovascularization Using Corticosteroid and Intravitreal Ranibizumab. Biomed Res Int. 2018 Sep 13;2018:1585803. doi: 10.1155/2018/1585803. PMID: 30302336; PMCID: PMC6158959.
- ↑ https://euretina.org/resource/abstract_2021_fluocinolone-acetonide-intravitreal-implant-0-19mg-in-the-management-of-punctate-inner-choroidopathy/
- ↑ Mangat SS, Ramasamy B, Prasad S, Walters G, Mohammed M, Mckibbin M. Resolution of choroidal neovascularization secondary to punctate inner choroidopathy (PIC) with intravitreal anti-VEGF agents: a case series. Semin Ophthalmol. 2011 Jan;26(1):1-3.
- ↑ Vossmerbaeumer U, Spandau UH, V Baltz S, Wickenhaeuser A, Jonas JB. Intravitreal bevacizumab for choroidal neovascularisation secondary to punctate inner choroidopathy. Clin Experiment Ophthalmol. 2008 Apr;36(3):292-4.
- ↑ Tarantola RM, Folk JC, Boldt HC, Mahajan VB.Intravitreal bevacizumab during pregnancy. Retina. 2010 Oct;30(9):1405-11.
- ↑ Jump up to: 49.0 49.1 Rouvas A, Petrou P, Douvali M, Ntouraki A, Vergados I, Georgalas I, Markomichelakis N. Intravitreal ranibizumab for the treatment of inflammatory choroidal neovascularization. Retina. 2011 May;31(5):871-9.
- ↑ Goff MJ, Johnson RN, McDonald HR, Ai E, Jumper JM, Fu A. Intravitreal bevacizumab for previously treated choroidal neovascularization from age-related macular degeneration. Retina. 2007 Apr-May;27(4):432-8.
- ↑ Brouzas D, Charakidas A, Rotsos T, Moschos MM, Loukianou H, Koutsandrea C, Ladas I, Baltatzis S. Choroidal neovascularization due to punctate inner choroidopathy: long-term follow-up and review of literature. Clin Ophthalmol. 2010 Aug 9;4:871-6.
- ↑ Rogers A, Duker J, Nichols N, Baker B. Photodynamic therapy of idiopathic and inflammatory choroidal neovascularization in young adults. Ophthalmology. 2003;110:1315–1320.
- ↑ Sickenberg M, Schmidt-Erfuth U, Miller JW, et al. A preliminary study of photodynamic therapy using verteporfin for choroidal neovascularization in pathologic myopia, ocular histoplasmosis syndrome, angioid streaks, and idiopathic causes. Arch Ophthalmol. 2000;117:327–336.
- ↑ Dimitrios Brouzas, Antonios Charakidas, Tryfon Rotsos, Marilita M Moschos, Helen Loukianou, Chryssanthy Koutsandrea, Ioannis Ladas, and Stefanos Baltatzis. Choroidal neovascularization due to punctate inner choroidopathy: long-term follow-up and review of literature. Clin Ophthalmol. 2010; 4: 871–876.
- ↑ Wachtlin J, Heimann H, Behme T, Foerster MH. Long-term results after photodynamic therapy with verteporfin for choroidal neovascularizations secondary to inflammatory chorioretinal diseases. Graefes Arch Clin Exp Ophthalmol. 2003;241:899–906.
- ↑ Chatterjee S, Gibson JM. Photodynamic therapy: a treatment option in choroidal neovascularization secondary to punctate inner choroidopathy. Br J Ophthalmol. 2003;87:917–927.
- ↑ Postelmans L, Pasteels B, Coquelet P, et al. Photodynamic therapy for subfoveal classic choroidal neovascularization related to punctate inner choroidopathy (PIC) or presumed ocular histoplasmosis-like syndrome (POHS-like) Ocul Immunol Inflamm. 2005;13:361–366.
- ↑ Lim J, Flaxel C, LaBree L. Photodynamic therapy for choroidal neovascularization secondary to inflammatory chorioretinal disease. Ann Acad Med Singapore. 2006;35:198–202.
- ↑ Coco RM, de Souza CF, Sanabria MR. Photodynamic therapy for subfoveal and juxtafoveal choroidal neovascularization associated with punctate inner choroidopathy. Ocul Immunol Inflamm. 2007;15:27–29.
- ↑ Leslie T, Lois N, Christopoulou D, Olson JA, Forrester JV. Photodynamic therapy for inflammatory choroidal neovascularisation unresponsive to immunosuppression. Br J Ophthalmol. 2005 Feb;89(2):147-50
- ↑ Jump up to: 61.0 61.1 Spaide RF, Sorenson J, Maranan L. Combined photodynamic therapy and intravitreal triamcinolone for nonsubfoveal choroidal neovascularization. Retina. 2005 Sep;25(6):685-90.
- ↑ Ehlers JP, Maldonado R, Sarin N, Toth CA. Treatment of non-age-related macular degeneration submacular diseases with macular translocation surgery. Retina. 2011 Jul-Aug;31(7):1337-46.
- ↑ Pachydaki SI, Jakobiec FA, Bhat P, Sobrin L, Michaud NA, Seshan SV, D'Amico DJ. Surgical management and ultrastructural study of choroidal neovascularization in punctate inner choroidopathy after bevacizumab. J Ophthalmic Inflamm Infect. 2012 Mar;2(1):29-37. doi: 10.1007/s12348-011-0050-x. Epub 2011 Nov 27. PubMed PMID: 22120962; PubMed Central PMCID: PMC3302998.
- ↑ Buerk BM, Rabb MF, Jampol LM. Peripapillary subretinal fibrosis: a characteristic finding of multifocal choroiditis and panuveitis. Retina. 2005;25(2):228e9
- ↑ Morgan CM, Schatz H. Recurrent multifocal choroiditis. Ophthalmology. Sep 1986;93(9):1138-47.