Preservatives in Topical Ophthalmic Medications
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
Introduction
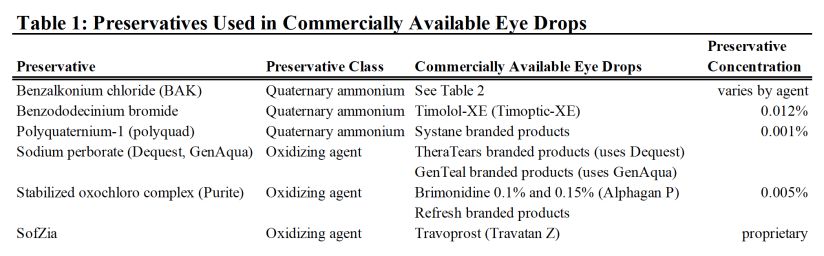
Preservatives provide important and necessary antimicrobial activity and are crucial in maintaining sterility and extending the shelf-life of multi-dose formulations of topical ophthalmic medications.[1] Their use has been a requirement in multi-dose eye drop formulations by many regulatory authorities since the 1970s including the United States Food and Drug Administration (US FDA), US Pharmacopeia (USP), and European Pharmacopoeia (EP).[2][3] In general preservatives can be classified into two main categories: detergents or oxidizing agents.[4] Many different molecules have antimicrobial properties and have been incorporated into various prescription and over the counter topical ophthalmic medications (Table 1).
However, preservatives can be toxic to the ocular surface, particularly in the setting of chronic, prolonged exposure, as in patients with glaucoma who may have therapeutic regimens that involve multiple eye drops and frequent instillation, or in patients with pre-existing ocular surface disease.[1] Of all ophthalmic preservatives, benzalkonium chloride (BAK) is most commonly used and most widely studied, with many studies demonstrating ocular surface toxicity.[1][2][3][4] As a result, a number of alternative classes of preservatives have been developed, all with differing efficacy profiles and degrees of ocular surface toxicity.
Benzalkonium chloride (BAK)
BAK is used in approximately 70% of topical ophthalmic eyedrops (Table 2).[1] As a preservative, BAK is used in concentrations from 0.003-0.02%.[1] BAK is a quaternary ammonium compound with both hydrophilic and hydrophobic properties that allows it to be highly hydrosoluble.[1] BAK acts as a detergent, disrupting the lipid component of microbial cell walls resulting in cell lysis.[2][3] It is an effective bactericidal agent with broad-spectrum efficacy against both gram-positive and gram-negative bacteria as well as against fungi and acanthamoeba.[1][3][5]
BAK has been proposed to enhance drug penetration into the cornea and subsequently the anterior chamber by disrupting the hydrophobic barrier of the corneal epithelium, breaking cell-cell junctions, and thus resulting in greater tissue and aqueous drug concentration and thereby improving efficacy.[3] Animal studies have shown improved transcorneal penetration of topical acyclovir[6] and gatifloxacin[7] when combined with BAK. When BAK is utilized as a preservative in antibiotic drops, the disruption of the corneal barrier by BAK may potentiate the antimicrobial effect of the “active ingredient” of the drop; additionally, the antimicrobial efficacy of BAK itself may also have a synergistic effect with the “active ingredient” antimicrobial.[8][9] Importantly, multiple studies have shown clinical equivalence between some BAK-preserved and unpreserved ophthalmic medications: notably, multiple trials comparing preserved and preservative-free formulations of different glaucoma medications found no difference in intraocular pressure (IOP) reduction.[10][11] Thus, in certain clinical scenarios, a change from a BAK-preserved to BAK-free regimen is often feasible without compromising IOP control.
The disruption to the corneal barrier may contribute to the ocular surface toxicity of BAK. The cytotoxic effects of BAK on ocular tissue cells (particularly conjunctival and corneal epithelium) has been well studied and documented. The threshold concentration at which toxicity from BAK occurs has been estimated to be 0.005% - much lower than many of its commercially used concentrations.[3] In vitro studies have demonstrated that BAK induces concentration-dependent decrease in cellular viability and has proapoptotic effects.[3] Animal studies have shown dose-dependent corneal epithelial damage and conjunctival inflammatory changes, findings which have been validated in human subjects.[3] BAK has been shown in patients to result in decreased goblet cell density which normally produce mucins and participate in tear film stability.[3] Additionally, BAK is also a detergent for the lipid layer of the tear film, increasing evaporation of the aqueous tear film, resulting in a dysfunctional, unstable tear film.[3] Both likely potentiate corneal damage and dry eye symptoms in BAK patients.
Symptoms of BAK toxicity can present in a spectrum from subclinical to severe. Clinical manifestations of BAK-induced surface disease include subjective discomfort and pain, increased tearing, increased fluorescein staining of the cornea and conjunctiva, lower Schirmer scores, worse tear film break up time (TBUT), increased incidence of superficial punctate keratitis, and worse scores on the Ocular Surface Disease Index (OSDI).[1][3] Cumulative burden of BAK exposure (number of medications, variable BAK concentration in each drop, drop frequency per day, duration of therapy) has been shown to correlate with ocular surface disease prevalence and severity in glaucoma patients[12] and worse quality of life[13].
Attempts have been made to improve drug tolerability by minimizing BAK’s toxic effects. Notably, once-daily formulations of medications like latanoprost and extended-release timolol function to decrease cumulative exposure to BAK.[3] Additionally, fixed-combination drops containing multiple medications may reduce cumulative exposure to BAK in comparison to use of the individual medications as separate drops (e.g. use of fixed combination dorzolamide-timolol eyedrop in comparison to use of both separate individual dorzolamide and timolol eyedrops).[3] Many newer drug formulations also utilize lower concentrations of BAK (≤0.005%) as studies have shown less corneal damage and conjunctival inflammation with lower concentration of BAK.[3] Moreover, some companies are moving towards eliminating BAK from medication formulations through addition of different preservatives and through preservative-free formulations. Additional strategies to reduce BAK-containing eye drop burden on the ocular surface in glaucoma patients include substitution with oral glaucoma medications (i.e. carbonic anhydrase inhibitors)[14], intracameral injection of long-acting glaucoma medications (Durysta bimatoprost intracameral implant [Allergan, Irvine, CA, USA])[15], and procedural interventions (e.g. laser trabeculoplasty, cyclophotocoagulation, or incisional glaucoma surgery).[14] These strategies may be utilized to sufficiently reduce IOP while simultaneously allowing for reduction in the number of topical medications.
Animal studies have also suggested possible fetal risk of BAK. In rats, a dose-related increase in resorptions and fetal death and a reduction in litter size and weight were observed in pregnant rats treated vaginally with BAK.[16] To mitigate theoretical BAK risk in pregnant humans, systemic absorption of BAK from eye drops can be mitigated using punctal occlusion and reduction in drop frequency.[17] Additionally, IOP tends to decrease during pregnancy which may facilitate reducing eyedrop burden.[18]
Polyquad (polyquaternium-1; PQ)
Polyquad (PQ)is found in contact lens solutions and artificial tear formulations (notably Systane artificial tears [Alcon Laboratories, Inc., Fort Worth, TX, USA]). It was also previously used in a preparation of travoprost (that is no longer commercially available in the US) (Table 1). The typical concentration of PQ is 0.001%.[19]
PQ is a quaternary ammonium compound similar to BAK.[1] Unlike BAK it is primary hydrophilic and much larger in size, about 27 times larger than BAK.[1] Both its large size and lack of hydrophobic region may prevent it from entering mammalian cells, theoretically leading to less cytotoxicity and ocular surface disease compared to BAK.[1]
In vitro and animal comparison studies demonstrate a more favorable tolerability profile for PQ than BAK, including less cytotoxicity and apoptosis and greater viability of corneal and conjunctival cells.[19] Some clinical studies comparing PQ-preserved travoprost to BAK-preserved prostaglandins found lower rates of ocular surface disease and improved OSDI scores.[20][21] Rossi et al also found improved TBUT, decreased corneal staining, and decreased punctate keratitis.[20] However, not all comparative studies have found decreased ocular surface toxicity with polyquad preserved ophthalmic formulations.[22] And in vitro studies have shown that PQ-containing eye drops can decrease cell viability and activate NF-kB and other inflammatory markers demonstrating the potential cytotoxic effects of PQ-1.[23] In general, available comparison studies between polyquad and BAK-preserved medications are open-label, have small sample sizes, and have short follow-up durations. There are no randomized control trials. Clearly, more studies are needed to fully determine if polyquad formulated eye drops are truly safer for the ocular surface compared to BAK.
Purite® (stabilized oxychloro complex; SOC)
Purite is found in many Refresh branded artificial tears (Allergan, Irvine, CA, USA) as well as Alphagan P (Brimonidine 0.15%; Allergan, Irvine, CA, USA) (Table 1).
Purite or stabilized oxocloro complex (SOC) contains a mixture of chlorite, chlorate, and chlorine dioxide.[4] Purite is a type of oxidative preservative characterized by small molecules that penetrate cell membranes and disrupt normal cellular function.[24] In solution, chlorine dioxide free radicals impart its antimicrobial by oxidizing unsaturated lipids and glutathione in the cell.[4] It has effective antimicrobial capabilities against many types of bacteria as well as the fungus Aspergillus niger.[4] Once administered to the eye, Purite converts to natural tear components including sodium and chloride ions, oxygen, and water.[4] Additionally, Purite is thought to be less toxic against mammalian cells as unlike many microorganisms, mammalian cells are equipped with antioxidants, oxidases, and catalases to neutralize Purite’s oxidative effects.[24]
Compared to other preservatives, Purite appears to be much less toxic. Purite preserved brimonidine P compared to BAK-preserved glaucoma medications was shown to induce less corneal damage and conjunctival inflammatory changes in animal studies.[25] Another animal study comparing artificial tears preserved with Purite, Polyquad, and GenAqua found less corneal epithelial damage with Purite and GenAqua similar to untreated controls.[4] Studies comparing Alphagan P to BAK-preserved brimonidine found better tolerability in the Alphagan P group.[26][27]
SofZia®
SofZia is a proprietary preservative used in Travatan Z (travoprost 0.004%; Novartis, Basel, Switzerland) (Table 1).
SofZia is an ionic-buffered preservative that contains borate, sorbitol, propylene glycol, and zinc.[1] It functions as an oxidizing preservative[28] with antibacterial and antifungal properties.[1] The components quickly degrade upon contact with cations on the ocular surface resulting in less cytotoxicity compared to BAK.[1]
There have been many clinical studies on the improvement of the ocular surface of patients switched from BAK-preserved prostaglandin to travoprost preserved with SofZia. Studies have shown improvement in OSDI scores[29][30], improved TBUT[30], and decreased corneal staining[30] after switching to travoprost Z. A long-term study comparing SofZia-preserved travoprost and BAK-preserved latanoprost similarly found improvement in both corneal staining and conjunctival hyperemia.[31]
Sodium perborate (GenAqua® and Dequest®; SPB)
Sodium perborate (SPB) is utilized as a preservative in some ophthalmic lubricating drops. In Genteal artificial tears (Novartis Pharmaceuticals, East Hanover, NJ, USA), SPB is referred to as GenAqua, and in TheraTears artificial tears (Prestige Consumer Healthcare, USA), SPB is referred to as Dequest. (Table 1).
SPB is one of the first oxidative preservatives.[28] It acts by forming hydrogen peroxide when combined with water thus giving it potent antimicrobial properties.[28] It is an effective bactericidal agent with action against bacteria, viruses, and the fungus Aspergillus niger.[4][24] Once SPB is applied to the ocular surface, existing conjunctival enzymes such as catalase rapidly decompose it to oxygen and water, limiting its cytotoxicity.[4][28] In vitro studies demonstrate that SPB is significantly less toxic to corneal and conjunctival cells compared to BAK.[32]
Preservative-free medications
Select topical ophthalmic medications are commercially available in preservative-free formulations (Table 3).
When available, preservative-free products can prevent ocular surface toxicity and improve tolerability and accordingly patient adherence, particularly in predisposed patients with compromised epithelium. Additionally, in some disease states such as severe dry eye syndrome, neurotrophic keratopathy, and limbal stem cell deficiency, the use of preserved drops should be minimized.
Several large epidemiological studies have compared glaucoma patients on preserved and preservative-free eye drops and found that patients on preservative-free formulations had significantly reduced ocular symptoms including discomfort upon instillation, burning and stinging, foreign body sensation, and tearing, as well as objective ocular findings including superficial punctate keratitis, conjunctival hyperemia and follicles, and eyelid eczema.[33][34] The frequency of ocular symptoms and objective findings of ocular surface disease also appears to increase with the number of preserved drops utilized.[33] Accordingly, studies have found that glaucoma patients on multiple topical glaucoma medications and higher daily dose of BAK had worse OSDI and quality of life scores.[13] Additionally, studies have found that decreasing the number of preserved eye drops or switching from preserved to non-preserved formulations will significantly decrease ocular symptoms and improve the ocular surface.[33][34][35]
However, preservative-free medications are not without limitations. Preservative-free formulations tend to be more expensive than their preserved counterparts and may be subject to limited or no insurance coverage restricting access for many patients.[28] Additionally, most preservative-free products are packaged in single-dose vials meant to be discarded after each use to prevent contamination.[28] For patients who chronically use eye drops, often with multiple times a day dosing, like those with dry eye disease or glaucoma, the cost of these preservative-free medications can become a significant burden. Therefore, patients may try to save vials after first use in order to extract subsequent doses which can increase their risk of infection.[28] The single-dose vials may also be tricky for patients to use, for example, the small vial size can make gripping and squeezing more difficult compared to conventional larger, multi-dose bottles particularly if the patient has an underlying tremor or arthritis.[1]
Advances in bottle design have created multi-dose preservative free bottles (ABAK® and COMOD®) in an attempt to combat both cost and ease of use of single-dose preservative-free drops.[28] The ABAK® bottle (Laboratoires Thea, France) uses a bi-functional membrane with anti-microbial properties to maintain sterility for up to three months after opening.[28] The COMOD® bottle (Ursapharm, Germany) has a one-way valve which maintains sterility for up to six months after opening.[28] In the United States, a 2022-2023 outbreak of extensively drug-resistant Pseudomonas aeruginosa infections was linked to usage of preservative-free artificial tears produced in a multidose bottle.[36] The implicated artificial tear products were distributed as EzriCare Artificial Tears (Ezricare, LLC, Lakewood, NJ, USA) and Delsam Pharm Artificial Tears (Delsam Pharm, LLC, Bronx, NY, USA). An investigation by the United States Centers for Disease Control and Prevention is ongoing to determine contributing factors to the outbreak.[36]
Perhaps most limiting is that many topical therapies are not commercially available in preservative-free formulations. There are increasing numbers of compounding pharmacies that can make preservative-free versions of medications, however this often comes at an additional out-of-pocket cost to the patient. Medications produced by compounding pharmacies are not approved by the US FDA. A full discussion of regulations on compounding of medications is beyond the scope of this article, and can be found on the FDA website.[37]
Conclusion
Preservatives are important additives in multi-dose topical ophthalmic medications. BAK, the most commonly used ophthalmic preservative, has been shown to cause ocular surface toxicity. Patients on multiple preservative-containing eye drops or with pre-existing ocular surface disease may benefit from decreasing cumulative BAK exposure. Switching to once-daily or fixed combinations of different medications, newer non-BAK preserved medications, or preservative-free medications or considering the addition of oral medications or long-acting intracameral inserts or procedural interventions may reduce patient symptoms and intolerability and improve the ocular surface.
References
- ↑ 1.00 1.01 1.02 1.03 1.04 1.05 1.06 1.07 1.08 1.09 1.10 1.11 1.12 1.13 1.14 Goldstein MH, Silva FQ, Blender N, et al. Ocular benzalkonium chloride exposure: problems and solutions. Eye 2022;36:361–368.
- ↑ 2.0 2.1 2.2 Steven DW, Alaghband P, Lim KS. Preservatives in glaucoma medication. Br J Ophthalmol 2018;102:1497–1503.
- ↑ 3.00 3.01 3.02 3.03 3.04 3.05 3.06 3.07 3.08 3.09 3.10 3.11 3.12 3.13 Baudouin C, Labbé A, Liang H, et al. Preservatives in eyedrops: The good, the bad and the ugly. Progress in Retinal and Eye Research 2010;29:312–334.
- ↑ 4.0 4.1 4.2 4.3 4.4 4.5 4.6 4.7 4.8 Noecker R. Effects of common ophthalmic preservatives on ocular health. Adv Therapy 2001;18:205–215.
- ↑ Heaselgrave W, Hamad A, Coles S, Hau S. In Vitro Evaluation of the Inhibitory Effect of Topical Ophthalmic Agents on Acanthamoeba Viability. Transl Vis Sci Technol 2019;8:17.
- ↑ Majumdar S, Hippalgaonkar K, Repka MA. Effect of chitosan, benzalkonium chloride and ethylenediaminetetraacetic acid on permeation of acyclovir across isolated rabbit cornea. Int J Pharm 2008;348:175–178.
- ↑ Rathore MS, Majumdar DK. Effect of formulation factors on in vitro transcorneal permeation of gatifloxacin from aqueous drops. AAPS PharmSciTech 2006;7:57.
- ↑ Tu EY, Shoff ME, Gao W, Joslin CE. Effect of low concentrations of benzalkonium chloride on acanthamoebal survival and its potential impact on empirical therapy of infectious keratitis. JAMA Ophthalmol 2013;131:595–600.
- ↑ Kowalski RP, Kowalski BR, Romanowski EG, et al. The in vitro impact of moxifloxacin and gatifloxacin concentration (0.5% vs 0.3%) and the addition of benzalkonium chloride on antibacterial efficacy. Am J Ophthalmol 2006;142:730–735.
- ↑ Easty DL, Nemeth-Wasmer G, Vounatsos J-P, et al. Comparison of a non-preserved 0.1% T-Gel eye gel (single dose unit) with a preserved 0.1% T-Gel eye gel (multidose) in ocular hypertension and glaucomatous patients. Br J Ophthalmol 2006;90:574–578.
- ↑ Hamacher T, Airaksinen J, Saarela V, et al. Efficacy and safety levels of preserved and preservative-free tafluprost are equivalent in patients with glaucoma or ocular hypertension: results from a pharmacodynamics analysis. Acta Ophthalmol Suppl (Oxf ) 2008;242:14–19.
- ↑ Leung EW, Medeiros FA, Weinreb RN. Prevalence of Ocular Surface Disease in Glaucoma Patients. Journal of Glaucoma 2008;17:350–355.
- ↑ 13.0 13.1 Skalicky SE, Goldberg I, McCluskey P. Ocular Surface Disease and Quality of Life in Patients With Glaucoma. American Journal of Ophthalmology 2012;153:1-9.e2.
- ↑ 14.0 14.1 Ghahari E, Djalilian AR, Aref AA. Management of Glaucoma in Patients with Ocular Surface Disease. In: Djalilian AR, ed. Ocular Surface Disease. Cham: Springer International Publishing; 2018:125–138. Available at: http://link.springer.com/10.1007/978-3-319-15823-5_9 [Accessed February 13, 2023].
- ↑ Sirinek PE, Lin MM. Intracameral sustained release bimatoprost implants (Durysta). Semin Ophthalmol 2022;37:385–390.
- ↑ Buttar HS. Embryotoxicity of benzalkonium chloride in vaginally treated rats. J Appl Toxicol 1985;5:398–401.
- ↑ Kumari R, Saha BC, Onkar A, et al. Management of glaucoma in pregnancy - balancing safety with efficacy. Ther Adv Ophthalmol 2021;13:25158414211022876.
- ↑ Khong EWC, Chan HHL, Watson SL, Lim LL. Pregnancy and the eye. Curr Opin Ophthalmol 2021;32:527–535.
- ↑ 19.0 19.1 Rolando M, Crider JY, Kahook MY. Ophthalmic preservatives: focus on polyquaternium-1. Expert Opinion on Drug Delivery 2011;8:1425–1438.
- ↑ 20.0 20.1 Rossi GCM, Scudeller L, Rolle T, et al. From benzalkonium chloride-preserved Latanoprost to Polyquad-preserved Travoprost: a 6-month study on ocular surface safety and tolerability. Expert Opinion on Drug Safety 2015;14:619–623.
- ↑ El Hajj Moussa WG, Farhat RG, Nehme JC, et al. Comparison of Efficacy and Ocular Surface Disease Index Score between Bimatoprost, Latanoprost, Travoprost, and Tafluprost in Glaucoma Patients. Journal of Ophthalmology 2018;2018:1–7.
- ↑ Muz OE, Dagdelen K, Pirdal T, Guler M. Comparison of BAK-preserved latanoprost and polyquad-preserved travoprost on ocular surface parameters in patients with glaucoma and ocular hypertension. Int Ophthalmol 2021;41:3825–3835.
- ↑ Paimela T, Ryhänen T, Kauppinen A, et al. The preservative polyquaternium-1 increases cytoxicity and NF-kappaB linked inflammation in human corneal epithelial cells. Mol Vis 2012;18:1189–1196.
- ↑ 24.0 24.1 24.2 Kaur IP, Lal S, Rana C, et al. Ocular preservatives: associated risks and newer options. Cutan Ocul Toxicol 2009;28:93–103.
- ↑ Noecker RJ, Herrygers LA, Anwaruddin R. Corneal and conjunctival changes caused by commonly used glaucoma medications. Cornea 2004;23:490–496.
- ↑ Mundorf T, Wilcox KA, Ousler GW, et al. Evaluation of the comfort of Alphagan® P compared with Alphagan® in irritated eyes. Adv Therapy 2003;20:329–336.
- ↑ Katz LJ. Twelve-Month Evaluation of Brimonidine-Purite Versus Brimonidine in Patients With Glaucoma or Ocular Hypertension: Journal of Glaucoma 2002;11:119–126.
- ↑ 28.0 28.1 28.2 28.3 28.4 28.5 28.6 28.7 28.8 28.9 Walsh K, Jones L. The use of preservatives in dry eye drops. OPTH 2019;Volume 13:1409–1425.
- ↑ Stewart J. Efficacy, safety, and improved tolerability of travoprost BAK-free ophthalmic solution compared with prior prostaglandin therapy. OPTH 2008:613.
- ↑ 30.0 30.1 30.2 Kahook M. Effects of prostaglandin analog therapy on the ocular surface of glaucoma patients. OPTH 2009:291.
- ↑ Aihara M, Otani S, Kozaki J, et al. Long-term Effect of BAK-free Travoprost on Ocular Surface and Intraocular Pressure in Glaucoma Patients After Transition From Latanoprost. Journal of Glaucoma 2012;21:60–64.
- ↑ Epstein SP, Ahdoot M, Marcus E, Asbell PA. Comparative Toxicity of Preservatives on Immortalized Corneal and Conjunctival Epithelial Cells. Journal of Ocular Pharmacology and Therapeutics 2009;25:113–119.
- ↑ 33.0 33.1 33.2 Pisella PJ, Pouliquen P, Baudouin C. Prevalence of ocular symptoms and signs with preserved and preservative free glaucoma medication. Br J Ophthalmol 2002;86:418–423.
- ↑ 34.0 34.1 Jaenen N, Baudouin C, Pouliquen P, et al. Ocular symptoms and signs with preserved and preservative-free glaucoma medications. Eur J Ophthalmol 2007;17:341–349.
- ↑ Uusitalo H, Egorov E, Kaarniranta K, et al. Benefits of switching from latanoprost to preservative-free tafluprost eye drops: a meta-analysis of two Phase IIIb clinical trials. OPTH 2016:445.
- ↑ 36.0 36.1 Centers for Disease Control and Prevention. (2023, February 9). Outbreak of extensively drug-resistant pseudomonas aeruginosa associated with artificial tears. Centers for Disease Control and Prevention. Retrieved February 13, 2023, from https://www.cdc.gov/hai/outbreaks/crpa-artificial-tears.html
- ↑ Center for Drug Evaluation and Research. (2020, September 10). Compounding laws and policies. U.S. Food and Drug Administration. Retrieved February 13, 2023, from https://www.fda.gov/drugs/human-drug-compounding/compounding-laws-and-policies