PreserFlo Ab-Externo MicroShunt
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
Background
Intraocular pressure (IOP) lowering is the only proven strategy for decreasing the risk of progression and associated irreversible vision loss in glaucomatous individuals. Medical, laser, and surgical therapies may be employed for this purpose. The surgical landscape for glaucoma includes several novel techniques and devices. Glaucoma surgeries that filter aqueous into the subconjunctival and sub-Tenon spaces include trabeculectomy, aqueous shunt surgery, the Xen Glaucoma Treatment System (Abbvie/Allergan Co), and the PreserFlo Ab-Externo Microshunt (Santen, Inc).
The PreserFlo Ab-Externo Microshunt is CE-marked in Europe and FDA approval in the United States is pending.
Device
The PreserFlo is composed of poly(styrene-block-isobutylene-block-styerene) or SIBS. This material is uniquely biologically inert and has been used in coronary stents. The properties of this material should theoretically decrease the risk for postoperative episcleral scarring and fibrosis, which are often causes of surgical failure after glaucoma filtering procedures.
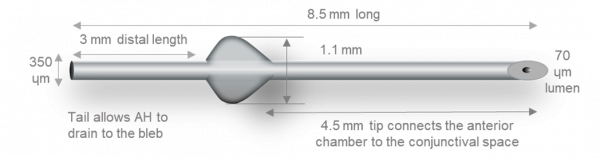
The device has a total length of 8.5 mm, which is divided by a 1mm “fin” into distal (3 mm) and proximal (4.5 mm) segments. The external lumen is 350um and the internal lumen is 70um with a beveled tip at the proximal end.
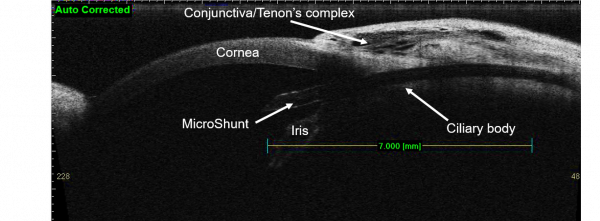
The design of the device obeys assumptions of the Hagen-Poiseuille equation for the prediction of pressure. Therefore, providing that aqueous production is > 2uL/min, postoperative IOP should be maintained above 5 mmHg. When positioned properly, the distal end of the MicroShunt should filter aqueous to the subconjunctival and sub-Tenon space 6 mm posterior to the limbus, allowing for posteriorly directed flow and bleb formation.
Indications
The PreserFlo Ab-Externo MicroShunt is CE-marked in Europe and FDA approval in the United States is currently pending.
The device is indicated for lowering of IOP in order to decrease the risk of progressive glaucoma and associated irreversible vision loss. Cases where the device may be implanted for this purpose may include open angle glaucomas such as primary open angle glaucoma (POAG), pseudoexfoliation glaucoma, pigmentary glaucoma, or other glaucomas with an open anterior chamber angle.
Contraindications and Risk Factors
Contraindications to implantation of the PreserFlo Ab-Externo Microshunt may include cases of shallow anterior chamber, inability of the patient to adhere to postoperative visits and/or medications, and/or intolerance or allergy to Mitomycin-C (MMC).
Surgical Technique
The device may be implanted under topical, local, or general anesthesia. After informed consent and standard ophthalmic prepping and ocular exposure, a 3 to 4 clock-hour fornix-based conjunctival peritomy is created – typically in the superonasal or superotemporal quadrants. Care should be taken to disinsert Tenon capsule attachments at the limbus and wide, blunt dissection should be carried out under this layer of tissue. Hemostasis may be performed as necessary using standard methods. Mitomycin-C soaked sponges are then applied to the scleral bed. Mitomycin-C concentration and duration of applications are left to the surgeon’s discretion based on judgment of risk for postoperative fibrosis. After removal of the sponges, the scleral bed is rinsed with balanced salt solution. The sclera is then marked 3 mm posterior the limbus. A scleral tunnel is initiated at this point along the curvature of the globe, followed by change trajectory at the level of the trabecular meshwork before entering the anterior chamber parallel to the iris plane. Different techniques and tools may be used to perform this portion of the procedure. The device is then inserted through this tract using non-toothed forceps, with care to maintain the proximal tip in a bevel-up position. The device is advanced until the fins are just tucked within the distal end of the scleral tunnel. In this position, 2 to 3 mm of the proximal end of the device should extend into the mid-anterior chamber and parallel to iris plane.
When properly positioned and unobstructed, aqueous flow should be visible at the distal end of the device. If no flow is visible and an obvious proximal obstruction is not visualized, the distal end may be “primed” using an anterior chamber cannula and balanced salt solution. A corneal paracentesis may be created at surgeon discretion for additional control of the anterior chamber or to test device patency via injection of balanced salt solution. Once the device has been placed and flow has been verified, conjunctiva and Tenon capsule are repositioned over the device, taking care to avoid distal obstruction of implant. The distal end of the implant should lie flush with the sclera and a second instrument may be used to direct it in this manner. The conjunctiva and Tenon capsule are then closed using the surgeon’s preferred technique. A fluorescein strip is used to confirm adequate wound closure and the eye is protected according to the surgeon’s usual protocol.
Postoperatively, the patient is treated with a topical antibiotic and steroid regimen with dosing frequency and duration based on examination findings and surgeon judgment.
Outcomes
Surgical outcomes with the PreserFlo Ab-Externo MicroShunt have been reported in a number of studies and publications.
Batlle and colleagues[1] reported on three-year outcomes in a prospective study of 23 patients that underwent the procedure at a single site. Fourteen of the patients underwent standalone MicroShunt implantation and 9 patients underwent the procedure in combination with cataract surgery. Importantly, patients received MMC at a concentration of .4mg/mL for minutes intraoperatively. The study group defined complete and qualified surgical success using standard definitions where complete success required a postoperative IOP < 21 mmHg, reduction from baseline by >20%, no reoperation for glaucoma, no loss of light perception vision, and no hypotony defined as IOP <5 mmHg on 2 consecutive visits after 3 months as well as no use of supplemental medications. A qualified surgical success met these measures but required the use of supplemental medications. Among all patients, IOP was reduced from a baseline of 23.8+5.3 mmHg to 10.7+3.5 mmHg at 3 years postoperatively, representing a 55% reduction from baseline. The mean number of glaucoma medications per patient was reduced from 2.4+0.9 to 0.7+1.1 at 3 years postoperatively. A qualified surgical success rate (using a 14 mmHg rather than 21 mmHg threshold) was attained in 95% of patients at 3 years. Complications included transient hypotony in 3 patients and transient choroidal effusion in 2 patients. These events resolved spontaneously in all patients. One patient failed the procedure due to persistent elevation in IOP attributed to bleb encapsulation. Another patient required needling of an encapsulated bleb to regain IOP control and another patient experienced proximal tube obstruction with fibrin which was relieved with anterior chamber irrigation.
In a retrospective case series, Schlenker et al[2] reported on outcomes in 164 consecutive eyes of 132 patients that underwent implantation of the PreserFlo Ab-Externo MicroShunt in standalone fashion. At one year, 76.9% of eyes had achieved complete surgical success (IOP > 6 and < 17 mmHg with no vision loss > 2 lines due to hypotony, no medication usage, IOP reduction > 20% from baseline, no reoperations performed in operating room, and no loss of light perception vision) and 92.5% achieved qualified success. A multivariate analysis was performed to assess risk factors for failure. The group found that an MMC dose of 0.2 versus 0.4-0.5 mg/mL (hazard ratio 2.51; 95% CI 1.12-5.65), as well as a diagnosis of secondary open-angle glaucoma versus POAG (hazard ratio 2.51; 95% CI 1.01-6.23) were independent risk factors for surgical failure. Postoperative bleb needling was required in 8.5% of eyes. Two eyes required surgical revision and one eye required reoperation for glaucoma. No eyes failed due to clinical hypotony. Transient complications included choroidal detachments, hyphemas, and anterior chamber shallowing.
Beckers and colleagues[3] reported on outcomes with the device in a prospective, multicenter study of 81 patients with mild-to-severe POAG. Mitomycin-C was applied at 0.2 to 0.4 mg/mL concentrations. Overall mean IOP was reduced from 21.7+3.4 to 14.1 mmHg at 2 years (P<0.0001) with an overall success rate of 74.1%. Six patients required reoperations and 5 required bleb needling at 2 years of follow-up. In a post-hoc analysis, patients receiving 0.4 mg/mL of MMC were more likely to be medication-free compared to patients receiving 0.2 mg/ml (90.3% versus 51.9%, respectively; P=0.001).
Baker and colleagues[4] reported on one-year results from a 2-year, prospective, randomized, multicenter trial in which patients with mild-to-severe POAG were randomized, 3:1, to undergo stand-alone MicroShunt implantation (395 patients) versus trabeculectomy (132 patients) surgery. All patients in both study groups received MMC at a concentration of 0.2 mg/mL for 2 minutes. At year 1, IOP decreased from 21.1+4.9 mmHg to 14.3+4.3 mmHg in the MicroShunt group versus 21.1+5.0 mmHg to 11.1 +4.3 mmHg in the trabeculectomy group. The probability of surgical success was higher in patients randomized to trabeculectomy versus the MicroShunt (72.7% vs. 53.9%, P<0.01). Medication usage was significantly reduced in both treatment arms. The incidence of transient hypotony was greater in patients randomized to trabeculectomy versus the MicroShunt (49.6% vs. 28.9%, P<0.01).
In a retrospective study conducted by Ibarz Barbera and colleagues[5], they evaluated the efficacy of PreserFlo Ab-Externo Microshunt in 64 POAG eyes. Of which, 51 underwent PreserFlo alone and 14 underwent PreserFlo combined with cataract surgery. MMC was applied at 0/2 mg/mL concentration. All eyes completed a minimum follow-up of 9 months. They reported a statistically significant reduction in the mean IOP from 22.03±0.7 mmHg on a mean of 2.7±0.7 medications preoperatively to 12.7±0.4 mmHg on a mean of 0.2±0.5 medications at the last follow-up. The success rates, defined as IOP between 6 and 17 mmHg with IOP reduction of at least 20% from the baseline line without (complete success) or with (qualified success) glaucoma medications, were 70.3% for complete success and 12.5% for qualified success at the final follow-up.
In refractory childhood glaucoma, Brandt[6] prospectively investigated the safety and efficacy of PreserFlo Microshunt in 12 eyes with a mean IOP of 22.7±4.8 mmHg on 3.3±0.6 medications at baseline. All 12 eyes had at least one year of follow-up. At the last follow-up, the success rate, defined as ≥25% IOP reduction without (complete) or with (qualified) glaucoma medications and with clinical evidence of response, was 75% (9 eyes) with a mean postoperative IOP of 11.0±2.5 mmHg in the success group.
A recent study found comparable IOP-lowering efficacy with the Preserflo compared to trabeculectomy in the intermediate term.[7]
Conclusion
The PerserFlo Ab-Externo MicroShunt is a novel device designed to reduce IOP while minimizing the risk of postoperative hypotony and vision-threatening hypotony-related complications. Published efficacy and safety outcomes with the device are encouraging and suggest that higher concentrations of intraoperative MMC may be associated with higher rates of surgical success. The device is CE-marked for use in Europe and FDA approval in the United States is pending.
References
- ↑ Batlle JF, Fantes F, Riss I, et al. Three-Year Follow-up of a Novel Aqueous Humor MicroShunt. J Glaucoma 2016;25:e58-e65.
- ↑ Schlenker MB, Durr GM, Michaelov E, Ahmed IIK. Intermediate Outcomes of a Novel Standalone Ab Externo SIBS Microshunt with Mitomycin C. Am J Ophthalmol 2020; 215:141-153.
- ↑ Beckers HJM, Aptel F, Webers CAB, et al. Safety and Effectiveness of the PRESERFLO MicroShunt in Primary Open-Angle Gluacoma: Results from a 2-Year Multicenter Study. Ophthalmol Glaucoma 2021 Jul27;S2589-4196(21)00179-4.
- ↑ Baker ND, Barnebey HS, Moster MR, et al. Ab-Externo MicroShunt versus Trabeculectomy in Primary Open-Angle Glaucoma: One-Year Results from a 2-Year Randomized, Multicenter Study. Ophthalmology. 2021 May27;S0161-6420(21)00384-5.
- ↑ Ibarz Barberá, Marta MD, PhD*,†; Martínez Galdón, Fátima OD*,†; Caballero Magro, Elena OD*,†; Rodríguez-Piñero, Marta MD*,†; Tañá Rivero, Pedro MD, PhD‡,§ Efficacy and Safety of the Preserflo Microshunt ® with Mytomicin C for the Treatment of Open Angle Glaucoma, Journal of Glaucoma: May 17, 2022 - Volume - Issue - 10.1097/IJG.0000000000002052
- ↑ Brandt, James D. Use of a Novel Microshunt in Refractory Childhood Glaucoma: Initial Experience in a Compassionate Use/Early Access Cohort. Am J Ophthalmolo, Volume 239, 223 - 229
- ↑ Gubser PA, Pfeiffer V, Hug S, Shang X, Lincke JB, Häner NU, Zinkernagel MS, Unterlauft JD. PRESERFLO MicroShunt implantation versus trabeculectomy for primary open-angle glaucoma: a two-year follow-up study. Eye Vis (Lond). 2023 Dec 21;10(1):50. doi: 10.1186/s40662-023-00369-8. PMID: 38124210; PMCID: PMC10734133.