Pre-Ophthalmologist Management of Eye Trauma
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
Introduction
The initial evaluation of ocular trauma is often performed by a non-ophthalmologist. Triage is focused on addressing life-threatening and then vision-threatening conditions. Adopting a methodological approach for triage of ocular trauma helps non-ophthalmologists identify vision-threatening conditions and optimize patient outcomes.
Epidemiology
A study by the Agency for Healthcare Research and Quality estimated that in 2008 about 640,000 emergency department visits were due to eye injury, a rate of 209 visits per 100,000 people.[1] The incidence of ocular injury is high in children, peaks in the third decade, and finally declines with age. McGwin et al found that most injuries occur at home (44.6%) and are either contusions or corneal abrasions (44.4%).[2] Although most ocular injuries are minor, it is important for the triaging provider to recognize vision-threatening conditions that require prompt ophthalmology consultation.
Injury Classification
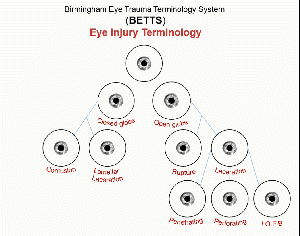
Mechanical globe injuries are classified as open or closed. Open globes involve a full thickness injury to the cornea or sclera and are caused by either sharp lacerations or blunt ruptured globe. Sub-classifications of lacerations include penetrating – single laceration of the eye wall; perforating – two full thickness lacerations due to an injury with an entry and an exit wound; and intraocular foreign body – a laceration where the inciting insult was retained within the eye as a foreign object. Closed globe injuries do not involve a full thickness injury, and can be classified as contusions or lamellar lacerations.[3] This standard classification schema can be helpful in evaluating and documenting globe injuries, but can also be useful in determining urgency of referral and prognosis.
In addition, open globes can be categorized based on zone of injury. Zone I injuries affect the cornea up to the limbus, Zone II injuries affect the limbus to the area 5mm back within the sclera, and Zone III affects the sclera beyond 5mm back. This classification is useful to understand the surgical approach as well as prognosis.
There are also injuries to the orbit and adnexa requiring prompt attention. Orbital fractures can involve any of the bony walls of the orbit, and can result in open or closed fractures. In younger patients, concerns for entrapped extraocular muscles occur due to the relative elasticity of young bone, which is considered a surgical emergency. Eyelid lacerations can also occur, affecting the skin and soft tissues. If the laceration is full thickness and affects the margin of the eyelid or the canalicular system, results in fat prolapse from the orbit, or causes avulsion of the lid, it should be promptly evaluated by an ophthalmologist.
Initial Approach
The assessment of an ocular trauma patient begins with addressing any life-threatening injuries. Once medically stable, identifying vision-threatening conditions requires a focused ocular history: injury mechanism, setting and timing of injury, ocular review of systems and past ocular history including pre-injury visual acuity.[4] These details aid in diagnosis and affect medical decision making. Other relevant past medical history includes: current medications, drug allergies, prior surgeries, complications with anesthesia, and tetanus immunization status.[4] Patients with potential open globe should have nausea and vomiting controlled as well as an eye shield placed for protection. Manipulation of the eye should be avoided, and any patient with a visible foreign body partially in the eye and/or orbit should not have it removed by a non-ophthalmologist.
Next, visual acuity (VA), relative afferent pupillary defect (rAPD), and confrontational visual field testing should be checked in both eyes.[5] A study by Rao et al found that presenting VA of <5/200 was the most important negative prognostic factor in open globe injuries.[6] In a patient with rAPD, the affected eye dilates during the swinging light test and may suggest optic nerve injury, vitreous hemorrhage, or retinal detachment.[7] If an open globe injury is excluded, intraocular pressure (IOP) should be measured. Pressures between 11 mmHg and 21 mmHg are considered normal. In the setting of trauma, hyphema, traumatic glaucoma, and orbital compartment syndrome can cause elevated IOP while low pressures are worrisome for an open globe injury.[8] [9] [10]
Pre-ophthalmologic Management of Threats to Vision
Chemical Injury
The severity of ocular damage from chemicals depends on the type of agent, volume, and duration of exposure. Alkaline agents saponify fatty acids of the corneal membrane, causing liquefactive necrosis that allows for deep intraocular penetration. The damage can reach limbal stem cells and prevent normal healing. Acid injuries cause coagulative necrosis from denatured proteins protecting the eye from deeper acid penetration.[11] [12] Because time between chemical exposure and irrigation has the most influence on prognosis, irrigation with isotonic saline or lactate ringer solution should be performed immediately, prior to ocular evaluation.[13] Irrigation should continue until pH testing at the conjunctival fornix with litmus paper confirms a pH between 7.0 to 7.4. Once physiologic pH is maintained, the eye can be further examined for corneal abrasions, globe rupture, foreign bodies, and damage to the exterior structures of the eye. As with mechanical injuries, visual acuity and IOP should be tested.[12] Topical anesthetics can attenuate pain to optimize patient comfort.[13] Furthermore, a Morgan Lens is commonly used to provide effective, continuous irrigation despite blepharospasms.[14] Occasionally, retained foreign material in the fornix can continue to leech out alkali or acidic material and result in an intractable change in pH until the inciting material is removed.
Orbital Fractures
Blunt trauma most often cause fractures to the orbital floor and medial orbital wall.[15] The mechanism is explained by both the buckling and hydraulic theory where force is transmitted through the bone and globe, respectively.[16] Understanding relevant facial anatomy helps correlate clinical findings and guide management. The orbital floor is comprised of the maxillary, zygomatic, and palatine bones. Entrapment of the inferior rectus muscle or adjacent soft tissue can restrict vertical eye movement and cause diplopia. If damage occurs to the infraorbital nerve, which traverses the floor of the orbit, there may be altered sensation in the area of the ipsilateral inferior orbital rim to the upper lip.[15]
The medial wall is comprised of the frontal process of the maxilla, the lacrimal bone, the orbital plate of the ethmoid bone, and the sphenoid body. Entrapment of the medial rectus can restrict horizontal eye movement.[17] Examination may reveal retraction of the globe and narrowing of the palpebral fissure on attempted abduction (Pseudo-Duane’s Retraction Syndrome).[18] Medial wall trauma can damage the nasolacrimal drainage system and medial canthal tendon resulting in epiphora (an overflow of tears onto the face) and widened intercanthal distance, respectively.[19] [20]
Forced duction test can identify extraocular muscle entrapment.[21] Thin cut CT scan of the orbit with axial and coronal views is the imaging modality of choice because it can detect involvement with the optic canal, presence of orbital hemorrhage, and visualize entrapment of the rectus muscles.[19] Any patient with limited upgaze should have concern for entrapment, even if not visualized on CT. Patients should avoid blowing their nose in the acute period to decrease the risk of orbital emphysema. Steroids may decrease orbital edema and antibiotics may be considered to prevent orbital cellulitis secondary to communication between the orbit and paranasal sinuses in cases with significant sinus disease.[19] Surgical intervention is considered for the following: persistent diplopia, clinical evidence of entrapment (positive forced duction test or oculocardiac reflex), enophthalmos >2 mm 14 days after trauma, and a fracture that involves more than 50% of the orbital floor.[19]
Orbital Compartment Syndrome
Because the orbit is an enclosed space, a rise in volume from an acute hemorrhage (laceration of infraorbital or ethmoid arteries) or soft tissue swelling can lead to a rapid increase in intraorbital pressure as well as IOP. Compartment syndrome occurs when intraorbital pressure exceeds arterial perfusion pressure of the optic nerve, resulting in permanent vision loss within 90 minutes.[22] Orbital compartment syndrome is a clinical diagnosis and should be suspected in patients presenting with trauma, double vision, or proptosis with signs of decreased VA, rAPD, ophthalmoplegia, periocular edema, and evidence of increased orbital pressure such as tense eyelids and resistance to retropulsion. Cutting the lateral canthal tendon along its axis (lateral canthotomy) and severing the inferior portion of the tendon (inferior cantholysis) provide emergent decompression which is demonstrated by a rapid lowering of the IOP.[10]
Open Globe Injury
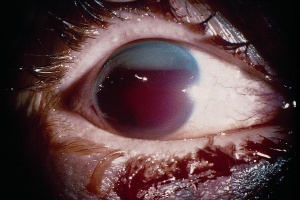
A full thickness injury to the cornea or sclera results in an open globe. The patient may present with sudden eye pain and vision loss after trauma. Examination may show decreased visual acuity, an irregular-shaped pupil, deepening or shallowing of the anterior chamber, extrusion of vitreous with loss of eyeball contour, and ocular hypotony.[4][23] In order to prevent extrusion of intraocular content, maneuvers that increase IOP, such as tonometry and eyelid retraction, should be avoided. Removal of foreign objects should be deferred, and the affected eye protected with an eye shield. The patient should be given antiemetics, pain medication, and remain NPO on bed rest with the head of bed elevated to 30 degrees.[24] Midazolam is a good option for its anxiolytic, sedative properties and lack of rise in IOP.[25] The patient’s tetanus should be up to date and broad-spectrum antibiotics administered to decrease the risk of endophthalmitis.[24] High dose systemic steroids may prevent Sympathetic Ophthalmia, a bilateral, granulomatous uveitis that follows exposure to neo-antigens after trauma. However this entity is rare, and steroids are not generally utilized for prevention at time of injury.[23][26]
Traumatic Hyphema
Trauma is one of many causes of hyphema, the presence of blood in the anterior chamber. Ocular contusion causes antero-posterior (AP) compression of the globe with simultaneous equatorial elongation, stretching and damaging the seven rings of trauma. This expansion can damage the iris or ciliary body vessels with subsequent bleeding.[27] [28] Classification is useful for prognosis and management; they are graded from 0 - IV based on the amount of blood present in the anterior chamber on slit lamp examination. Visual prognosis is usually worse with higher grades, but it is also dependent on etiology and development of complications.[29] Complications include obstruction of trabecular meshwork with increased IOP, optic atrophy, rebleeding, synechiae, and corneal blood staining.[28]
Initial management includes elevating the head of bed to 30 degrees, placement of an eye shield on the affected eye, and bed rest. Although, many studies find there are no advantages of bed rest versus ambulatory management, this decision is provider dependent.[28] NSAIDs and aspirin products should be avoided for pain control because their platelet inhibiting properties may increase bleeding.[30] Nausea and vomiting should be controlled to avoid increases in IOP. In the absence of narrow angle glaucoma, a mydriatic-cycloplegic such as atropine 1% can attenuate photophobia and stabilize the blood aqueous barrier. Topical prednisolone acetate 1% four times daily can decrease IOP, synechiae development, and risk of secondary bleeding.[28] The risks and benefits of discontinuing anticoagulation should be discussed with an internist. Sickle cell screening should be considered in African-American patients or other suspect individuals.[28] Inpatient management is recommended for hyphema with grade III or IV, bleeding coagulopathy, or sickle cell comorbidity. Patient adherence to activity limitation, medications, and ophthalmology follow up are vital to outpatient success; There should be a low threshold for hospital admission if the clinician suspects compliance issues.[28]
Retinal Detachment
Retinal detachment is the separation of the neurosensory retina (NSR) from the underlying retinal pigmented epithelium (RPE). These two layers are normally opposed by two forces: mechanical (interdigitation of microvilli) and metabolic (fluid flowing from the vitreous to the choroid). Ocular contusion causes the globe to compress with subsequent rebound decompression in the AP direction. These movements cause the vitreous to pull on the retina and may lead to retinal breaks. In addition, trauma may liquefy the vitreous (vitreous syneresis). The combination of a retinal break and liquefied vitreous can lead to accumulation of subretinal fluid with subsequent retinal detachment.[31] Patients may complain of new onset flashes, floaters, and visual field defects. Physical exam may show decreased visual acuity, loss of a red reflex, or rAPD. Direct ophthalmoscopy may visualize a retinal detachment but has low sensitivity due to its narrow field of view.[32] Use of a slit lamp can visualize brown pigmented cells in the anterior vitreous (Shafer’s sign or “tobacco dust”).[33] In patients with a closed globe and an obscured view of the retina, point-of-care ultrasonography using an ophthalmic setting can assist in diagnosis with high sensitivity and specificity.[34] Retinal detachment requires immediate ophthalmology referral.
Traumatic Optic Neuropathy
Traumatic optic neuropathy (TON) is caused by direct or indirect injury. Direct TON occurs when a penetrating object directly injures the optic nerve, but this is rare due to the bony anatomy of the orbit. Indirect TON results when force from blunt trauma deforms the optic canal, leading to shearing of the optic nerve.[35] Swelling of the optic nerve can compress the neurovascular bundle and thereby exacerbate ischemia.[36] Clinical features of TON may include decreased visual acuity, rAPD (only present in unilateral or asymmetric TON), red color desaturation (dyschromatopsia), and visual field defects.[35] The current literature on management is conflicting and lacking prospective, randomized control trials. Although historically treated with high-dose corticosteroids or optic canal decompression surgery, the International Optic Nerve Trauma Study did not show benefit with either intervention.[37] High dose steroids are contraindicated in patients with intracranial hemorrhage, due to increased morbidity.
Eyelid Laceration
The eyelids have an important role in tear film distribution and drainage, protection of the globe, and cosmesis. An understanding of the anatomy is important to recognize when ophthalmology referral is appropriate.[38] The eyelid skin is very thin because of the absence of subcutaneous fat. Underneath the skin is the orbicularis muscle, which closes the eyelid and aids in tear drainage. The orbital septum is a thin fibrous layer that separates the anterior from the intra-orbital eyelid structures. This septum also connects the periosteum of the superior orbital rim to the levator palpebrae superioris aponeurosis. Orbital fat is found between the orbital septum and levator muscle. The tarsal plates are thick fibrous tissue that serve as the main structural component of the eyelids. The conjunctiva is the inner lining of the eyelids and is continuous over the sclera to the edge of the cornea. The nasolacrimal system is located medially and allows fluid to drain into the nose. The path of fluid in the nasolacrimal system flows in this order: punctum, canaliculus, lacrimal sac, and finally underneath the inferior turbinate via the nasolacrimal duct. [39]
Management of eyelid lacerations requires recognition of concomitant ocular injuries and differentiating between simple versus complex lacerations; this distinction is dependent on the character and location of injury.[39] Prior to lid repair, it is important to appreciate the extent of injury by examining for conditions such as corneal abrasions, foreign bodies, hyphemas, and open globes. In the presence of an open globe, manipulation of the lid should be deferred due to the risk of expulsion of intraocular contents.[24][39] Referral to ophthalmology is appropriate in the following situations: full-thickness lid laceration, laceration with orbital fat prolapse, laceration involving the lid margins or lacrimal system, and lid trauma with avulsion.[38] Injury to the medial canthus is suspicious for canalicular involvement and requires evaluation with probing and irrigation of the drainage system.[40] Canalicular damage is common in eyelid lacerations from dog bites due to penetrating trauma or shearing forces.[41] Management of dog bites includes: copious irrigation, debridement of devitalized tissue, closure of the wound, and prophylactic antibiotic that covers aerobes and anaerobes. Postexposure prophylaxis for rabies and tetanus may be considered.[42] [43] Superficial lacerations that are greater than 25% of the lid can be reapproximated with 6-0 silk or plain gut sutures. The repair should occur within 12 to 36 hours of initial injury for optimal outcomes.[38] [39]
References
- ↑ Owens PL, Mutter R. Emergency Department Visits Related to Eye Injuries, 2008: Statistical Brief #112. In: Healthcare Cost and Utilization Project (HCUP) Statistical Briefs. Rockville (MD)2006.
- ↑ McGwin G, Jr., Owsley C. Incidence of Emergency Department–Treated Eye Injury in the United States. Archives of Ophthalmology. 2005;123(5):662-666.
- ↑ Pieramici DJ, Sternberg P, Aaberg TM, et al. A System for Classifying Mechanical Injuries of the Eye (Globe). American Journal of Ophthalmology. 1997;123(6):820-831.
- ↑ 4.0 4.1 4.2 Conrad DR. Ocular Trauma: Principles and Practice, Ferenc Kuhn, Dante J. Pieramici. Thieme (2002). Canadian Journal of Ophthalmology. 2004;39(7):802.
- ↑ Harlan JB, Jr., Pieramici DJ. Evaluation of patients with ocular trauma. Ophthalmol Clin North Am. 2002;15(2):153-161.
- ↑ Rao LG, Ninan A, Rao KA. Descriptive study on ocular survival, visual outcome and prognostic factors in open globe injuries. Indian J Ophthalmol. 2010;58(4):321-323.
- ↑ Belliveau AP, Somani AN, Dossani RH. Pupillary Light Reflex. In: StatPearls. Treasure Island (FL): StatPearls Publishing; 2020.
- ↑ Machiele R, Motlagh M, Patel BC. Intraocular Pressure. In: StatPearls. Treasure Island (FL)2020.
- ↑ Dharmasena A, Park DY, Vishwanath M. Does high intraocular pressure exclude an open globe injury? Int J Ophthalmol. 2014;7(2):389-390.
- ↑ 10.0 10.1 Lima V, Burt B, Leibovitch I, Prabhakaran V, Goldberg RA, Selva D. Orbital compartment syndrome: the ophthalmic surgical emergency. Surv Ophthalmol. 2009;54(4):441-449.
- ↑ Singh P, Tyagi M, Kumar Y, Gupta KK, Sharma PD. Ocular chemical injuries and their management. Oman J Ophthalmol. 2013;6(2):83-86.
- ↑ 12.0 12.1 Bates A, Zanaboni A. Ocular Burns. In: StatPearls. Treasure Island (FL): StatPearls Publishing; 2020.
- ↑ 13.0 13.1 Kuckelkorn R, Schrage N, Keller G, Redbrake C. Emergency treatment of chemical and thermal eye burns. Acta Ophthalmologica Scandinavica. 2002;80(1):4-10.
- ↑ Eslani M, Baradaran-Rafii A, Movahedan A, Djalilian AR. The ocular surface chemical burns. J Ophthalmol. 2014;2014:196827-196827.
- ↑ 15.0 15.1 Koenen L, Waseem M. Orbital Floor (Blowout) Fracture. In: StatPearls. Treasure Island (FL): StatPearls Publishing; 2020.
- ↑ Waterhouse N, Lyne J, Urdang M, Garey L. An investigation into the mechanism of orbital blowout fractures. British Journal of Plastic Surgery. 1999;52(8):607-612.
- ↑ Thiagarajah C, Kersten RC. Medial wall fracture: an update. Craniomaxillofac Trauma Reconstr. 2009;2(3):135-139.
- ↑ Duane TD, Schatz NJ, Caputo AR. Pseudo-Duane's retraction syndrome. Trans Am Ophthalmol Soc. 1976;74:122-132.
- ↑ 19.0 19.1 19.2 19.3 Joseph JM, Glavas IP. Orbital fractures: a review. Clin Ophthalmol. 2011;5:95-100.
- ↑ Zapala J, Bartkowski AM, Bartkowski SB. Lacrimal drainage system obstruction: management and results obtained in 70 patients. Journal of Cranio-Maxillofacial Surgery. 1992;20(4):178-183.
- ↑ Kim HS, Jeong EC. Orbital Floor Fracture. Arch Craniofac Surg. 2016;17(3):111-118.
- ↑ Roth FS, Koshy JC, Goldberg JS, Soparkar CNS. Pearls of orbital trauma management. Semin Plast Surg. 2010;24(4):398-410.
- ↑ 23.0 23.1 Mishra A, Baranwal VK, Parihar JKS, Verma AK. Simple laceration wound of the eyelids? Always remember to look under the lids! Med J Armed Forces India. 2013;69(3):301-304.
- ↑ 24.0 24.1 24.2 Blair K, Alhadi SA, Czyz CN. Globe Rupture. In: StatPearls. Treasure Island (FL)2020.
- ↑ Carter K, Faberowski LK, Sherwood MB, Berman LS, McGorray S. A randomized trial of the effect of midazolam on intraocular pressure. J Glaucoma. 1999;8(3):204-207.
- ↑ Damico FM, Kiss S, Young LH. Sympathetic Ophthalmia. Seminars in Ophthalmology. 2005;20(3):191-197.
- ↑ Wilson FM. Traumatic hyphema. Pathogenesis and management. Ophthalmology. 1980;87(9):910-919.
- ↑ 28.0 28.1 28.2 28.3 28.4 28.5 Walton W, Von Hagen S, Grigorian R, Zarbin M. Management of Traumatic Hyphema. Survey of Ophthalmology. 2002;47(4):297-334.
- ↑ Papaconstantinou D, Georgalas I, Kourtis N, et al. Contemporary aspects in the prognosis of traumatic hyphemas. Clin Ophthalmol. 2009;3:287-290.
- ↑ Gharaibeh A, Savage HI, Scherer RW, Goldberg MF, Lindsley K. Medical interventions for traumatic hyphema. Cochrane Database Syst Rev. 2013;12(12):CD005431-CD005431.
- ↑ Ghazi NG, Green WR. Pathology and pathogenesis of retinal detachment. Eye. 2002;16(4):411-421.
- ↑ Kang HK, Luff AJ. Management of retinal detachment: a guide for non-ophthalmologists. BMJ. 2008;336(7655):1235-1240.
- ↑ Tanner V, Harle D, Tan J, Foote B, Williamson TH, Chignell AH. Acute posterior vitreous detachment: the predictive value of vitreous pigment and symptomatology. British Journal of Ophthalmology. 2000;84(11):1264.
- ↑ Lahham S, Shniter I, Thompson M, et al. Point-of-Care Ultrasonography in the Diagnosis of Retinal Detachment, Vitreous Hemorrhage, and Vitreous Detachment in the Emergency Department. JAMA Netw Open. 2019;2(4):e192162-e192162.
- ↑ 35.0 35.1 Jang SY. Traumatic Optic Neuropathy. Korean J Neurotrauma. 2018;14(1):1-5.
- ↑ Sarkies N. Traumatic optic neuropathy. Eye. 2004;18(11):1122-1125.
- ↑ Levin LA, Beck RW, Joseph MP, Seiff S, Kraker R. The treatment of traumatic optic neuropathy: the International Optic Nerve Trauma Study. Ophthalmology. 1999;106(7):1268-1277.
- ↑ 38.0 38.1 38.2 Brown DJ, Jaffe JE, Henson JK. Advanced Laceration Management. Emergency Medicine Clinics of North America. 2007;25(1):83-99.
- ↑ 39.0 39.1 39.2 39.3 Cochran ML, Czyz CN. Eyelid Laceration. In: StatPearls. Treasure Island (FL): StatPearls Publishing; 2020.
- ↑ Philadelphia APMMDMPH, Jurij R. Bilyk MD. A Practical Approach to Canalicular Lacerations.
- ↑ Savar A, Kirszrot J, Rubin PAD. Canalicular involvement in dog bite related eyelid lacerations. Ophthalmic Plast Reconstr Surg. 2008;24(4):296-298.
- ↑ Nagendran S, Litwin AS, Sira M, Norris J, Malhotra R, Dheansa B. Management of facial and periocular dog bites: a review of 104 cases. European Journal of Plastic Surgery. 2014;37(11):595-598.
- ↑ Stevens DL, Bisno AL, Chambers HF, et al. Practice Guidelines for the Diagnosis and Management of Skin and Soft Tissue Infections: 2014 Update by the Infectious Diseases Society of America. Clinical Infectious Diseases. 2014;59(2):e10-e52.