Prader-Willi Syndrome
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
Disease Entity
Disease
Prader-Willi Syndrome
- ICD-10-CM Q87.11 Prader-Willi Syndrome
Etiology
Prader-Willi syndrome is caused by the lack of expression of paternally inherited genes in the 15q11.2-13 region on the long arm of chromosome 15. This can occur due to either deletions from the paternal chromosome or maternal disomy[2][3]. The vast majority of cases are sporadic rather than familial[4].
Epidemiology
Prader-Willi syndrome affects approximately 1 in 25,000 live births, with males and females affected equally.
Currently, Prader-Willi syndrome affects about 350,000 - 400,000 individuals worldwide[5]. Prevalence estimates differ among studies, however it is likely due to different testing methods rather than an increased risk in specific populations or countries.
The risk of recurrence in siblings is very low unless a deletion affecting the imprinting center is present, accounting for less than 1 percent of cases[6].
Pathophysiology
Prader-Willi Syndrome occurs with the loss of the paternal copy of chromosome region 15q11-q13[7]. The process by which gene expression occurs depends on the sex of the parent donating the gene is called genomic imprinting[7]. Loss of the maternal copy of 15q11-q13, in contrast, manifests as Angelman syndrome, a related but otherwise distinct disease entity[7].
Deletion of the paternal copy of 15q11-q13 is found in 70% of cases of Prader-Willi[7]. Alternatively, 25% of cases are associated with maternal uniparental disomy 15[7]. Imprinting defects and other chromosome 15 abnormalities account for the remaining cases[7].
The 15q11-q13 region is home to about 100 nonredundant genes or transcripts, and among these 12 or more are imprinted and paternally expressed[7]. Paternally expressed genes are candidates for causing Prader-Willi Syndrome while the imprinted maternally expressed genes UBE3A and ATP10A are candidates for causing Angelman syndrome[7]. Kemp et al. succinctly described the relationship of genomic imprinting as follows: “a child must receive the paternally derived gene at 15q12 to not develop Prader-Willi syndrome and must receive the maternally derived gene at 15q12 to not develop Angelman syndrome[8]." Paternally expressed genes in the 15q11-q13 region include SNURF-SNRPN, NDN, MKRN3, MAGEL2, and multiple copies of small untranslated nucleolar RNAs (snoRNAs or SNORDs)[7]. Genes in this region are known to have effects on axonal nerve growth, circadian rhythm, behavior, and fertility[7].
Clinical Features
Systemic Manifestations
The Neonate
- Neonatal hypotonia is a classic feature of this disorder, which can lead to asphyxia. These infants can have feeding difficulties, leading to failure to thrive and a weak cry [3][4].
- Genital hypoplasia[9][10]
- Hypopigmentation of the skin, iris, and hair[9][10].
The Toddler
- Delayed major motor milestones, such as walking at an average of 27 months and talking at an average of 39 months[4].
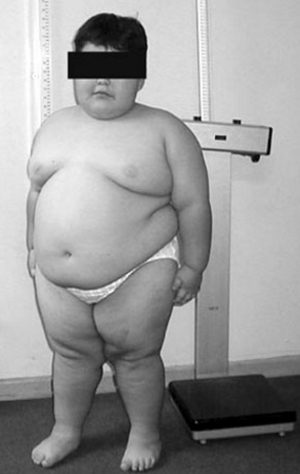
- Hyperphagia with subsequent obesity.
- Abnormal body composition with increased fat mass and reduced lean body mass compared with normal and obese controls[11][12].
- Resting energy use is also reduced lower than normal[13].
Childhood/Adolescence
- Evidence of growth hormone deficiency: short stature, failure to have a pubertal growth spurt with secondary sexual characteristics delayed or incomplete [14] [15].
- Central and obstructive sleep apnea, (50–100%). [16]
- Seizure disorder (26%) [17]
- Obesity complications such as Diabetes mellitus and Atherosclerosis
- Scoliosis[18][19].
- Behavioral problems and learning difficulties similar to those found in autism spectrum disorder are commonly seen in Prader-Willi syndrome[20]. Patients can also exhibit rectal gouging and skin-picking behavior that may respond to N-acetylcysteine treatment[21].
- Mood disorders and psychotic states among other psychiatric symptoms and disorders have been reported among adults[22].
- Cognitive disability (100%), ranging from mild to severe.[23]
- Abnormal food-seeking behaviors among Prader-Willi syndrome patients include stealing food, eating garbage, and eating frozen food.
- Decreased vomiting ability and increased pain tolerance have been associated with Prader-Willi syndrome[24].
Ocular Manifestations
Commonly reported ocular findings of patients with Prader-Willi syndrome include: [25] [26]
- Decreased visual acuity
- Iris and choroid hypopigmentation. There is an overlap between Prader-Willi Syndrome and oculocutaneous albinism that is attributed to the deletion of the OCA2 gene found in the PWS critical region[26].
- Refractive error: Vision Survey in the Global PWS Registry found that 41% of patients had myopia, 25% had hyperopia, 25% had astigmatism, and 16% had amblyopia[27].
- Strabismus. Vision Survey in the Global PWS Registry found that the prevalence of strabismus of Prader-Willi syndrome is 40%, and that 91% of those with strabismus were diagnosed before 5 years old. 42% of Prader-Willi syndrome patients with strabismus had strabismus surgery[27].
- Cataracts
- Congenital ocular fibrosis syndrome
- Diabetic retinopathy
- Nystagmus
- Congenital ectropion uveae
Prader-Willi-Like Syndromes
MAGEL2 (Melanoma Antigen L2) is one of the protein-coding genes found in the Prader-Willi region (15q11-q13) that is imprinted maternally. When the paternal alleles in this region are not expressed, it leads to the development of Prader-Willi syndrome. Conversely, mutations in the paternal allele of MAGEL2 that result in non-sense or frameshift changes are expected to produce a shortened protein and have been associated with Schaaf-Yang Syndrome (SYS) (Prader-Willi-like syndromes).[28][29] [30][31]Patients affected by SYS and PWS display similar clinical characteristics, including neonatal hypotonia, intellectual disability, developmental delay, early feeding difficulties, endocrinological disturbances, and sleep disorders. Nevertheless, certain clinical features used to diagnose PWS, such as hypopigmentation, distinctive facial abnormalities, small hands and feet, excessive appetite, obesity, and obsessive-compulsive behaviors, are often absent in SYS patients. Instead, SYS patients commonly exhibit more severe intellectual disability, behaviors associated with autism spectrum disorder, and joint contractures. [31][32]
While MAGEL gene-associated syndromes may share some common clinical features, they can have different genetic mutations or alterations within the MAGEL gene or associated genes. These genetic differences can result in variations in the severity of symptoms, associated medical complications, and potential long-term outcomes for individuals affected by these syndromes. Accurate diagnosis and differentiation of specific MAGEL gene-associated syndromes can help guide healthcare professionals in future research and potential targeted therapies for these syndromes providing appropriate medical care, management strategies, and genetic counseling for affected individuals and their families.
| MAGEL (Melanoma antigen L2) associated syndromes | Genetics | Systemic manifestations | Ocular manifestations |
| Schaaf-Yang syndrome (SYS) (Prader-Willi-like syndrome) or Chitayat Hall syndrome | Non-sense or frameshift mutations in the paternal allele of MAGEL2 encode a truncated protein[31][32][33] | Patients diagnosed with SYS and PWS (Prader-Willi syndrome) display similar clinical features, including neonatal hypotonia, intellectual disability (ID), developmental delay, early feeding difficulties, endocrinological imbalances, and sleep disorders. However, there are some differences between the two disorders. Notably, patients with SYS often lack some of the specific diagnostic criteria associated with PWS, such as hypopigmentation, distinct facial dysmorphisms, small hands and feet, hyperphagia, obesity, and obsessive-compulsive behaviors. In contrast, patients with SYS more frequently exhibit severe intellectual disability, autism spectrum disorder (ASD) behaviors, and joint contractures.[31] [33][34][35][36][37][38][39][40][41][42] | Exotropia, myopia,
strabismus, microcornea, microphthalmos, keratoconus, ptosis, and coloboma.[29][34][42][43] |
| Severe Arthrogryposis syndrome/Arthrogryposis Multiplex Congenita (AMC) | Truncating mutations in MAGEL2, deletions, and frameshift mutations in MAGEL2 have been identified as one of the known heterozygous genetic mutations associated with AMC.[44] | Polyhydramnios limited fetal movement, and contractures in the extremities are observed, along with congenital hypopituitarism. [44][45] | Severe visual impairment, optic nerve hypoplasia, astigmatism, divergent squint, and nystagmus. Other related conditions include congenital ophthalmoplegia, progressive bilateral paralysis of the lateral recti, delayed development of ocular movement, delayed maturation of fixation, microphthalmia, hypertelorism, lid abnormalities, optic atrophy, juvenile-onset glaucoma, Mobius syndrome, and Duane's syndrome.[46] |
| Opitz- C syndrome | One case report shows a de novo nonsense mutation in the MAGEL2 gene. [47] | Psychomotor delay, trigonocephaly, and a distinct set of facial abnormalities such as a receding chin, upward slanting of the eyes, and extra folds of skin near the inner corners of the eyes. Additionally, patients may exhibit a shortened neck, limited joint mobility, undescended testes, structural defects in the heart, and complications involving the pancreas and kidneys. Patients with this syndrome typically experience low muscle tone, joint stiffness, and seizures. | Retinitis pigmentosa, strabismus, exotropia, myopia, and coloboma.[47][48] |
Diagnosis
Prader-Willi syndrome is diagnosed after a thorough clinical evaluation resulting in multiple characteristic features and later confirmed with genetic testing[49].Major clinical features that are consistent with the diagnosis of PWS include hypotonia, developmental delay, hypothalamic hypogonadism, short stature and obesity[50].
The gold standard of genetic testing is methylation analysis (detects 99% of cases), which depends on the differences in methylation patterns in maternally or paternally inherited alleles in the 15q11-q13 region[4]. Fluorescent in-situ hybridization is another effective test however it is limited to the detection of Prader-Willi syndrome caused by a deletion (up to 75% of cases)[51][52].
Newer techniques such as chromosomal microarrays, genotyping with DNA markers, and methylation-specific multiplex ligation-dependent probe amplification (MS-MLPA) may also be used for diagnosis and characterization[7].
Management
Prader-Willi often requires multidisciplinary management due to the various pathologies that result from this condition. Treatment options also differ with age. While the management of hypotonia is the main concern for neonates and infants, obesity is the cornerstone of disease management in older patients[53]. Weight loss surgery is a method that has been used in the management of the pathologies associated with obesity in PWS but the results have been far from consistent[54][55].
Management of PWS also includes the screening and treatment of abnormal hypothalamic and pituitary function, growth hormone deficiency, hypogonadism, osteoporosis, hypothyroidism, adrenal insufficiency and sleep apnea[15][56][57][58].
Compared to the general population, the Prader-Willi syndrome population in the Global PWS Registry had considerably higher prevalence of strabismus, amblyopia, and hyperopia[27]. Therefore, it is critical to screen Prader-Willi syndrome patients for ocular issues.
Prognosis
The life expectancy for patients with Prader-Willi syndrome has been gradually increasing with advances in treatment techniques with many patients living beyond the age of 50 years[59][60][61]. The main causes of morbidity and mortality in patients with PWS include respiratory failure, cardiovascular disease, diabetes mellitus and intellectual disability[59][62][63].
Additional Resources
- Prader-Willi Syndrome Association: https://www.pwsausa.org
- Foundation For Prader-Willi Research: https://www.fpwr.org
- National Institutes of Health: Genetic and Rare Diseases Information Center: https://rarediseases.info.nih.gov/diseases/5575/prader-willi-syndrome
- National Organization for Rare Disorders: https://rarediseases.org/rare-diseases/prader-willi-syndrome/
References
- ↑ Cortés M F, Alliende R MA, Barrios R A, et al. Caracterización Clínico-Genético-molecular de 45 Pacientes Chilenos Con Síndrome de Prader willi. Revista médica de Chile. 2005;133(1). doi:10.4067/s0034-98872005000100005
- ↑ Lionti T, Reid SM, White SM, Rowell MM. A population-based profile of 160 Australians with Prader-Willi syndrome: trends in diagnosis, birth prevalence and birth characteristics. Am J Med Genet A 2015; 167A:371
- ↑ 3.0 3.1 Whittington JE, Butler JV, Holland AJ. Changing rates of genetic subtypes of Prader-Willi syndrome in the UK. Eur J Hum Genet 2007; 15:127
- ↑ 4.0 4.1 4.2 4.3 Butler MG. Prader-Willi syndrome: current understanding of cause and diagnosis. Am J Med Genet. 1990 Mar;35(3):319-32. doi: 10.1002/ajmg.1320350306. PMID: 2309779
- ↑ Butler MG, Hanchett JM, Thompson T. Clinical findings and natural history of Prader-Willi syndrome. In: Management of Prader-willi Syndrome, Butler MG, Lee PDK, Whitman BY (Eds), Springer, New York 2006
- ↑ Buiting, K, Horsthemke, B. Molecular Genetic Findings in Prader-willi Syndrome in Management of Prader-willi Syndrome, Butler MG, Lee PDK, Whitman BY (Eds), Springer, New York 2006. p.63
- ↑ 7.00 7.01 7.02 7.03 7.04 7.05 7.06 7.07 7.08 7.09 7.10 Butler MG. Prader-Willi and Angelman Syndromes: Examples of Genomic Imprinting. In: Murray MF, Babyatsky MW, Giovanni MA, Alkuraya FS, Stewart DR. eds. Clinical Genomics: Practical Applications in Adult Patient Care, 1e. McGraw Hill; 2014. Accessed April 06, 2022
- ↑ Chapter 6. Genetic Disorders. In: Kemp WL, Burns DK, Brown TG. eds. Pathology: The Big Picture. McGraw Hill; 2008. Accessed April 06, 2022
- ↑ 9.0 9.1 Butler MG. Hypopigmentation: a common feature of Prader-Labhart-Willi syndrome. Am J Hum Genet. 1989 Jul;45(1):140-6. PMID: 2741944; PMCID: PMC1683374.
- ↑ 10.0 10.1 Cassidy SB, Schwartz S, Miller JL, Driscoll DJ. Prader-Willi syndrome. Genet Med. 2012 Jan;14(1):10-26. doi: 10.1038/gim.0b013e31822bead0. Epub 2011 Sep 26. PMID: 22237428.
- ↑ Brambilla P, Bosio L, Manzoni P, Pietrobelli A, Beccaria L, Chiumello G. Peculiar body composition in patients with Prader-Labhart-Willi syndrome. Am J Clin Nutr. 1997 May;65(5):1369-74. doi: 10.1093/ajcn/65.5.1369. PMID: 9129464.
- ↑ Eiholzer U, Blum WF, Molinari L. Body fat determined by skinfold measurements is elevated despite underweight in infants with Prader-Labhart-Willi syndrome. J Pediatr. 1999 Feb;134(2):222-5. doi: 10.1016/s0022-3476(99)70419-1. PMID: 9931533.
- ↑ van Mil EA, Westerterp KR, Gerver WJ, Curfs LM, Schrander-Stumpel CT, Kester AD, Saris WH. Energy expenditure at rest and during sleep in children with Prader-Willi syndrome is explained by body composition. Am J Clin Nutr. 2000 Mar;71(3):752-6. doi: 10.1093/ajcn/71.3.752. PMID: 10702169.
- ↑ Whitman BY. Neurodevelopmental and neuropsychological aspects of Prader-Willi syndrome. In: Management of Prader-Willi Syndrome, 3rd ed, Butler M, Lee PDK, Whitman BY (Eds), Springer-Verlag, New York 2006. p.264
- ↑ 15.0 15.1 Burman P, Ritzén EM, Lindgren AC. Endocrine dysfunction in Prader-Willi syndrome: a review with special reference to GH. Endocr Rev. 2001;22(6):787-799. doi:10.1210/edrv.22.6.0447
- ↑ Miller J, Silverstein J, Shuster J, Driscoll DJ, Wagner M. Short-term effects of growth hormone on sleep abnormalities in Prader-Willi syndrome. J Clin Endocrinol Metab. 2006 Feb;91(2):413-7. doi: 10.1210/jc.2005-1279. Epub 2005 Nov 29. PMID: 16317059.
- ↑ Ergun-Longmire B, Nguyen MHN, Com G. Electrical status epilepticus during sleep in a child with Prader-Willi syndrome: a case report. AME Case Rep. 2022 Jan 25;6:7. doi: 10.21037/acr-21-34. PMID: 35128315; PMCID: PMC8762383.
- ↑ Vendrame M, Maski KP, Chatterjee M, Heshmati A, Krishnamoorthy K, Tan WH, Kothare SV. Epilepsy in Prader-Willi syndrome: clinical characteristics and correlation to genotype. Epilepsy Behav. 2010 Nov;19(3):306-10. doi: 10.1016/j.yebeh.2010.07.007. Epub 2010 Aug 21. PMID: 20727826.
- ↑ de Lind van Wijngaarden RF, de Klerk LW, Festen DA, Hokken-Koelega AC. Scoliosis in Prader-Willi syndrome: prevalence, effects of age, gender, body mass index, lean body mass and genotype. Arch Dis Child. 2008 Dec;93(12):1012-6. doi: 10.1136/adc.2007.123836. Epub 2008 Feb 8. PMID: 18263693.
- ↑ Bennett JA, Germani T, Haqq AM, Zwaigenbaum L. Autism spectrum disorder in Prader-Willi syndrome: A systematic review. Am J Med Genet A. 2015 Dec;167A(12):2936-44. doi: 10.1002/ajmg.a.37286. Epub 2015 Aug 29. PMID: 26331980.
- ↑ Bonnot O, Cohen D, Thuilleaux D, Consoli A, Cabal S, Tauber M. Psychotropic treatments in Prader-Willi syndrome: a critical review of published literature. Eur J Pediatr. 2016 Jan;175(1):9-18. doi: 10.1007/s00431-015-2670-x. Epub 2015 Nov 19. PMID: 26584571.
- ↑ Sinnema M, Boer H, Collin P, Maaskant MA, van Roozendaal KE, Schrander-Stumpel CT, Curfs LM. Psychiatric illness in a cohort of adults with Prader-Willi syndrome. Res Dev Disabil. 2011 Sep-Oct;32(5):1729-35. doi: 10.1016/j.ridd.2011.02.027. Epub 2011 Mar 31. PMID: 21454045.
- ↑ Cassidy SB, Schwartz S, Miller JL, Driscoll DJ. Prader-Willi syndrome. Genet Med. 2012 Jan;14(1):10-26. doi: 10.1038/gim.0b013e31822bead0. Epub 2011 Sep 26. PMID: 22237428.
- ↑ Wharton RH, Wang T, Graeme-Cook F, Briggs S, Cole RE. Acute idiopathic gastric dilation with gastric necrosis in individuals with Prader-Willi syndrome. Am J Med Genet. 1997 Dec 31;73(4):437-41. doi: 10.1002/(sici)1096-8628(19971231)73:4<437::aid-ajmg12>3.0.co;2-s. PMID: 9415471.
- ↑ Libov AJ, Maino DM. Prader-Willi syndrome. J Am Optom Assoc. 1994 May;65(5):355-9. PMID: 8071507
- ↑ 26.0 26.1 Hamid, M.A., Mehta, M.C. & Kuppermann, B.D. Multimodal imaging in a patient with Prader–Willi syndrome. Int J Retin Vitr 4, 45 (2018). https://doi.org/10.1186/s40942-018-0147-6
- ↑ 27.0 27.1 27.2 Bohonowych, J.E., Vrana-Diaz, C.J., Miller, J.L. et al. Incidence of strabismus, strabismus surgeries, and other vision conditions in Prader-Willi syndrome: data from the Global Prader-Willi Syndrome Registry. BMC Ophthalmol 21, 296 (2021). https://doi.org/10.1186/s12886-021-02057-4
- ↑ Schaaf CP, Marbach F. Schaaf-Yang Syndrome. In: GeneReviews®. University of Washington, Seattle, Seattle (WA); 1993. PMID: 33570896
- ↑ 29.0 29.1 Schaaf CP, Marbach F. Schaaf-Yang Syndrome. 2021 Feb 11 [Updated 2021 Nov 4]. In: Adam MP, Mirzaa GM, Pagon RA, et al., editors. GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle; 1993-2023. Available from: https://www.ncbi.nlm.nih.gov/books/NBK567492/.
- ↑ Buiting K, Di Donato N, Beygo J, et al. Clinical phenotypes of MAGEL2 mutations and deletions. Orphanet J Rare Dis. 2014;9:40.
- ↑ 31.0 31.1 31.2 31.3 Schaaf, C. P., Gonzalez-Garay, M. L., Xia, F., Potocki, L., Gripp, K. W., Zhang, B., ... & Yang, Y. Truncating mutations of MAGEL2 cause Prader-Willi phenotypes and autism. Nature genetics, 2013; 45(11), 1405-1408.
- ↑ 32.0 32.1 Cheon, C. K. Genetics of Prader-Willi syndrome and Prader-Will-like syndrome. Annals of pediatric endocrinology & metabolism, 2016; 21(3), 126-135.
- ↑ 33.0 33.1 Jobling, R., Stavropoulos, D. J., Marshall, C. R et al. Chitayat-Hall and Schaaf-Yang syndromes: a common aetiology: expanding the phenotype of MAGEL2-related disorders. Journal of medical genetics, 2018; 55(5), 316-321.
- ↑ 34.0 34.1 Fountain, M. D., & Schaaf, C. P. Prader-Willi syndrome and Schaaf-Yang syndrome: neurodevelopmental diseases intersecting at the MAGEL2 gene. Diseases, 2016; 4(1), 2.
- ↑ Castilla-Vallmanya, L., Centeno-Pla, M., Serrano, M., Franco-Valls, H et al. Advancing in Schaaf-Yang syndrome pathophysiology: from bedside to subcellular analyses of truncated MAGEL2. Journal of Medical Genetics, 2023; 60(4), 406-415
- ↑ Enya, T., Okamoto, N., Iba, Y et al. Three patients with Schaaf–Yang syndrome exhibiting arthrogryposis and endocrinological abnormalities. American Journal of Medical Genetics Part A, 2018; 176(3), 707-711.
- ↑ Marbach, F., Elgizouli, M., Rech, M et al. The adult phenotype of Schaaf-Yang syndrome. Orphanet Journal of Rare Diseases, 2020; 15, 1-11.
- ↑ Fountain, M. D., Aten, E., Cho, M. T et al. The phenotypic spectrum of Schaaf-Yang syndrome: 18 new affected individuals from 14 families. Genetics in Medicine, 2017;19(1), 45-52
- ↑ Chitayat, D., Hall, J. G., Couch, R. M et al. Syndrome of mental retardation, facial anomalies, hypopituitarism, and distal arthrogryposis in sibs. American Journal of medical genetics, 1990; 37(1), 65-70.
- ↑ Wortmann, S. B., Rodenburg, R., Schwahn et al. Distal joint contractures, mental retardation, characteristic face and growth retardation: Chitayat syndrome revisited. Genetic counseling, 2007;18(1), 119.
- ↑ Smigiel, R., Basiak, A., Misiak, B et al. Panhypopituitary insufficiency in a patient with clinical diagnosis of Chitayat-Hall syndrome. Endokrynologia Polska, 2010; 61(3), 318-321
- ↑ 42.0 42.1 Rao, V., El-Alem, T., Aminu, K., et al. Chitayat–Hall syndrome: extending the clinical phenotype. Clinical Dysmorphology, 2013; 22(4), 156-160.
- ↑ Patak, J., Gilfert, J., Byler, M., et al. MAGEL2‐related disorders: A study and case series. Clinical genetics, 2019; 96(6), 493-505.
- ↑ 44.0 44.1 Mejlachowicz, D., Nolent, F., Maluenda, J., et al. Truncating mutations of MAGEL2, a gene within the Prader-Willi locus, are responsible for severe arthrogryposis. The American Journal of Human Genetics, 2015; 97(4), 616-620.
- ↑ Gregory, L. C., Shah, P., Sanner, J. R., et al. Mutations in MAGEL2 and L1CAM are associated with congenital hypopituitarism and arthrogryposis. The Journal of Clinical Endocrinology & Metabolism, 2019; 104(12), 5737-5750.
- ↑ Puri, P., Gupta, M., & Chan, J. Progressive ophthalmoplegia in arthrogryposis multiplex congentia. Eye, 2002;16(1), 86-88.
- ↑ 47.0 47.1 Urreizti, R., Cueto-Gonzalez, A. M., Franco-Valls, H., et al. A de novo nonsense mutation in MAGEL2 in a patient initially diagnosed as Opitz-C: similarities between Schaaf-Yang and Opitz-C syndromes. Scientific reports, 2017; 7(1), 1-7.
- ↑ Urreizti, R., Grinberg, D., & Balcells, S. C syndrome-what do we know and what could the future hold?. Expert Opinion on Orphan Drugs, 2019;7(3), 91-94.
- ↑ Goldstone AP, Holland AJ, Hauffa BP, Hokken-Koelega AC, Tauber M; speakers contributors at the Second Expert Meeting of the Comprehensive Care of Patients with PWS. Recommendations for the diagnosis and management of Prader-Willi syndrome [published correction appears in J Clin Endocrinol Metab. 2010 Dec;95(12):5465]. J Clin Endocrinol Metab. 2008;93(11):4183-4197. doi:10.1210/jc.2008-0649
- ↑ Gunay-Aygun M, Schwartz S, Heeger S, O'Riordan MA, Cassidy SB. The changing purpose of Prader-Willi syndrome clinical diagnostic criteria and proposed revised criteria. Pediatrics. 2001;108(5):E92. doi:10.1542/peds.108.5.e92
- ↑ Driscoll DJ, Miller JL, Schwartz S, et al. Prader-Willi Syndrome. 1998 Oct 6 [Updated 2017 Dec 14]. In: Adam MP, Ardinger HH, Pagon RA, et al., editors. GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle; 1993-2022. Available from: https://www.ncbi.nlm.nih.gov/books/NBK1330/
- ↑ Whittington JE, Butler JV, Holland AJ. Changing rates of genetic subtypes of Prader-Willi syndrome in the UK. Eur J Hum Genet. 2007;15(1):127-130. doi:10.1038/sj.ejhg.5201716
- ↑ Butler MG, Lee J, Manzardo AM, et al. Growth charts for non-growth hormone treated Prader-Willi syndrome [published correction appears in Pediatrics. 2015 May;135(5):946]. Pediatrics. 2015;135(1):e126-e135. doi:10.1542/peds.2014-1711
- ↑ Scheimann AO, Butler MG, Gourash L, Cuffari C, Klish W. Critical analysis of bariatric procedures in Prader-Willi syndrome. J Pediatr Gastroenterol Nutr. 2008;46(1):80-83. doi:10.1097/01.mpg.0000304458.30294.31
- ↑ Alqahtani AR, Elahmedi MO, Al Qahtani AR, Lee J, Butler MG. Laparoscopic sleeve gastrectomy in children and adolescents with Prader-Willi syndrome: a matched-control study. Surg Obes Relat Dis. 2016;12(1):100-110. doi:10.1016/j.soard.2015.07.014
- ↑ Pellikaan K, Rosenberg AGW, Kattentidt-Mouravieva AA, et al. Missed Diagnoses and Health Problems in Adults With Prader-Willi Syndrome: Recommendations for Screening and Treatment. J Clin Endocrinol Metab. 2020;105(12):e4671-e4687. doi:10.1210/clinem/dgaa621
- ↑ Miller J, Wagner M. Prader-Willi syndrome and sleep-disordered breathing. Pediatr Ann. 2013;42(10):200-204. doi:10.3928/00904481-20130924-10
- ↑ de Lind van Wijngaarden RF, Otten BJ, Festen DA, et al. High prevalence of central adrenal insufficiency in patients with Prader-Willi syndrome. J Clin Endocrinol Metab. 2008;93(5):1649-1654. doi:10.1210/jc.2007-2294
- ↑ 59.0 59.1 Einfeld SL, Kavanagh SJ, Smith A, Evans EJ, Tonge BJ, Taffe J. Mortality in Prader-Willi syndrome. Am J Ment Retard. 2006;111(3):193-198. doi:10.1352/0895-8017(2006)111[193:MIPS]2.0.CO;2
- ↑ Sinnema M, Schrander-Stumpel CT, Maaskant MA, Boer H, Curfs LM. Aging in Prader-Willi syndrome: twelve persons over the age of 50 years. Am J Med Genet A. 2012;158A(6):1326-1336. doi:10.1002/ajmg.a.35333
- ↑ Whittington JE, Holland AJ, Webb T. Ageing in people with Prader-Willi syndrome: mortality in the UK population cohort and morbidity in an older sample of adults. Psychol Med. 2015;45(3):615-621. doi:10.1017/S0033291714001755
- ↑ Butler, Merlin G., et al. "Causes of death in Prader-Willi syndrome: Prader-Willi Syndrome Association (USA) 40-year mortality survey." Genetics in Medicine 19.6 (2017): 635-64
- ↑ Proffitt, Jennifer, et al. "Contributing factors of mortality in Prader–Willi syndrome." American journal of medical genetics Part A 179.2 (2019): 196-205.

