Posterior Ischemic Optic Neuropathy
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
Introduction
Posterior Ischemic Optic Neuropathy (PION) is an acute optic neuropathy due to ischemia in the posterior (retrobulbar) portion of the optic nerve. PION is characterized clinically by acute, painless vision loss in one or both eyes, the presence of a relative afferent pupillary defect (RAPD) in unilateral or bilateral but asymmetric cases and a normal fundus (retrobulbar optic neuropathy) [1]. The vision loss typically occurs over hours but can worsen over days to weeks.
PION is most common in the vasculopathic age range (i.e., after age 50 years) but can occur in any age depending on risk factors [2]. Only 10% of optic nerve ischemia cases are due to PION compared to its counterpart, Anterior Ischemic Optic Neuropathy (AION) [3]. PION is distinguished from AION by the appearance of a typically normal optic nerve head in PION compared with a swollen optic disc in AION. PION is due to ischemia of the posterior optic nerve [1], which is supplied by only the pial capillary plexus [3]. PION can lead to variable but sometimes profound vision loss due to a lack of perfusion to the posterior segment of the optic nerve [2]. There are three main etiologies of PION: non-arteritic non-surgical PION, arteritic PION caused by giant cell arteritis (GCA), and perioperative non-arteritic PION [1].
Epidemiology and Pathogenesis
The posterior segment of the optic nerve is separated into the intraorbital, intracanalicular, and intracranial parts. The blood supply to these various sections involve contributions from many branches and arterial sources; therefore, it is not necessary that the pathology of PION be localized to any one artery or location. The intraorbital segment is supplied by both a peripheral centripetal blood supply formed by the pial plexus, and an axial centrifugal blood supply formed by branches of the central retinal artery [1]. The intracanalicular portion is only supplied by the peripheral centripetal system from the branches of the ophthalmic artery, and the intracranial part has only a pial vascular plexus to support it, coming from variable arterial branches.
Of the various causes of PION, three main groups have been classified: non-arteritic, arteritic (GCA), and perioperative [3].
- Non-arteritic PION cases are rare and usually secondary to small vessel vascular disease, and have been linked to multifactorial systemic diseases such as diabetes mellitus (P=0.014), hypertension (P=0.022) with or without hypertensive retinopathy[4], atherosclerosis, that along with hypertension could impair autoregulation mechanisms [5], glaucoma[4], or anecdotal causes like carotid artery dissection, carotid cavernous fistula [6], migraine (P=0.039), hemodialysis, or head injury. [1]
- Arteritic PION, much less common that non-arteritic AION, is seen in older patients and is due to GCA, commonly involving the posterior ciliary arteries (PCA), but can also be due to other orbital arteries [1]. Arteritic PION is characterized by variable but often severe vision loss and a smaller chance of visual recovery than non-arteritic, non-surgical PION [3].
- Perioperative PION patients tend to be younger in age than arteritic PION patients and may have profound bilateral vision loss (70% of cases), and worse visual outcomes [3], with NLP being the most common outcome in some retrospective studies[4]. Prexisting factors that have been related to Perioperative PION include: male sex, obesity, obstructive sleep apnea and amiodarone or PDE-5 inhibitor use.[4]
Perioperative PION is believed to be multifactorial, but some proposed risk factors include:
- Prolonged intraoperative arterial hypotension
- Postoperative anemia
- Increased intraocular pressure (IOP) [7]
- Facial swelling [2]. While intraoperative mean arterial pressure (MAP) can usually be maintained during surgical procedures, an increase in IOP can cause decreased ocular perfusion and therefore produce ischemia, since mean ocular perfusion pressure (MOPP) is equal to MAP – IOP [8].
Although any general surgical procedure can cause perioperative PION, spinal surgery is one of the most common causes of PION, that is usually bilateral. In these spinal cases, the use of the Wilson frame [9], deliberate hypotension to decrease intraoperative blood loss [1], and the use of general anesthesia with the prone position have been proposed as risk factors [8].
Other surgeries associated to PION include periocular surgery such as blepharoplasty[10], sinus surgery for cocaine induced necrotizing sinusitis[11], and orbital decompression for thyroid eye disease (in the contralateral orbit)[12], venous graft in extremities, heart bypass, radical neck dissection, hip surgery, thoracotomy for hemothorax, prostatectomy, and breast augmentation and abdominal liposuction[13].
It is hypothesized that ischemia occurs in pial plexus and extends to the optic nerve, involving the centripetal blood supply which could be affected during surgical interventions in close proximity to the optic chiasm[14], or direct ischemia may come from small meningeal-based arteries at the orbital apex.Anecdotal evidence of PION resulting from a bilateral lung transplantation has also been described [15].
More recently during the COVID19 pandemic, there have been few reports of non-arteritic PION associated to COVID19[16], although the vast majority of cases are identified to be Anterior ION.
The histopathological findings of PION vary from patient to patient, and the lack of precise details for its progression can be attributed to only a handful of histopathological reports, many of which only include a single patient evaluation [1]. On occasion, it is reported that there is optic nerve ischemia sparing in either the peripheral or the central segment, but cases of total loss of optic nerve axons and total infarction of the optic nerve also exist [1]. This variability is due to the aforementioned diverse blood supply patterns from the central retinal artery. Specifically, ischemia involving centrifugal vascular systems spare the central part of the nerve, while those involving centripetal blood supply systems spare the peripheral portion of the nerve [1]. The latter is more common in both arteritic and non-arteritic PION [1]. Microscopic examination may include acellularity among fibrovascular pial septae, mild hemorrhage, Gitter-cell infiltrate, and loss of myelin [5].
Ocular Manifestations
PION typically presents as unilateral or bilateral vision loss that is both sudden and painless, but progressive initially [1]. Surgical PION is differentiated in the fact that patients only discover the vision loss upon waking from the procedure, which may be many days post-operation. In these patients, PION tends to be bilateral, profound, and permanent [1].
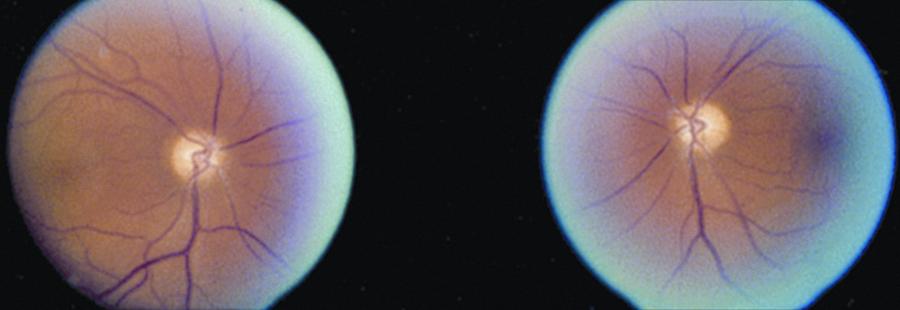
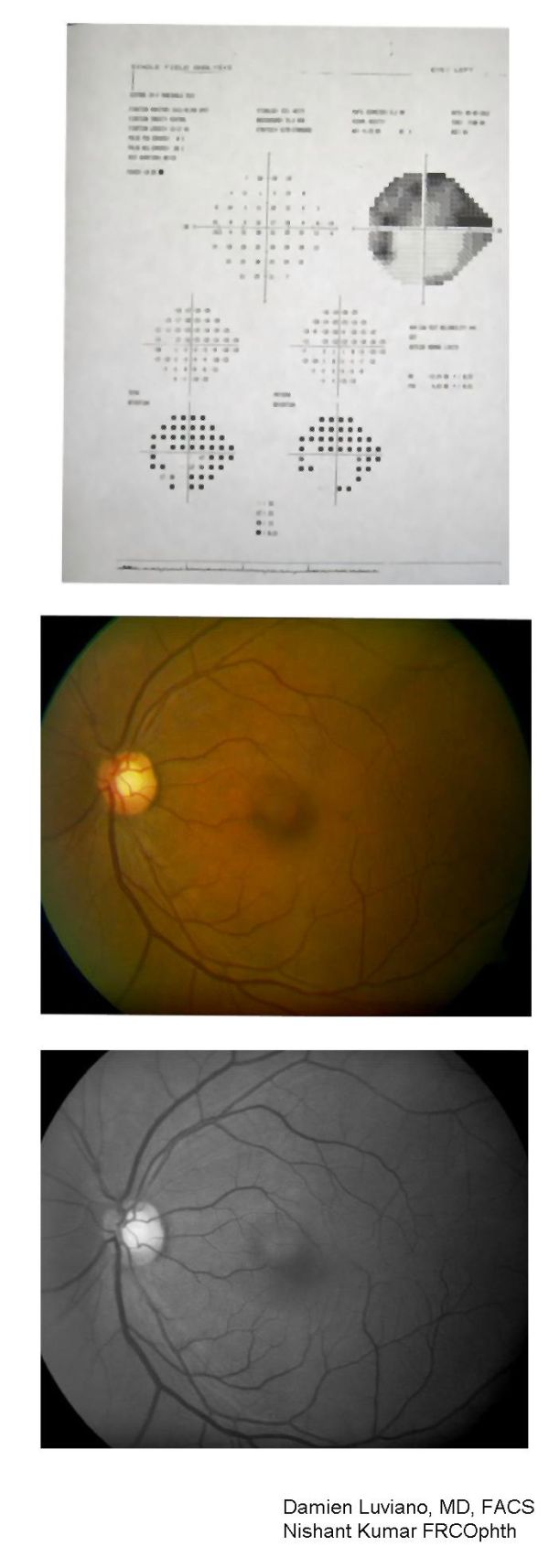
There are often no ocular abnormalities with the exception of RAPD in asymmetric PION cases. By ophthalmoscopy and fluorescein fundus angiography, there is a normal optic disc initially, but temporal optic disc pallor is common 6-8 weeks later [3] [1]. Absence of cup in the optic disc is not seen in the frequency with which it is present in AION [1]. Visual acuity ranges from normal to no light perception. In non-arteritic PION, acuity ranges from 20/25 or better in 17% of patients, 20/40 or better in 20%, and 20/200 or worse in 69% [17]. Central visual field defects are most common in both arteritic and non-arteritic PION cases [1]. However, they may also present with altitudinal defects. No alternative etiology for any ocular, orbital, or neurological findings should be found that could potentially explain patients’ vision loss in order to diagnose PION [1]. No disc edema will be present initially but over time optic atrophy develops in the affected eye.
Signs
In addition to vision loss, an RAPD is present in unilateral or bilateral but asymmetric PION cases. Otherwise, the anterior segment, intraocular pressure, and optic disc and fundus are normal until temporal pallor appears 6-8 weeks later. Post ischemic optic disc cupping may appear in arteritic or non-arteritic PION [17] and concomitant optic nerve atrophy with optic rim pallor is a distinguishing feature for PION related cupping compared with typical glaucomatous cupping [5].
Diagnosis
Principally, diagnosis of PION is a clinical diagnosis and is made by a process of exclusion.
The presence of acute vision loss, optic nerve-related visual field defects in the eye with vision loss, a RAPD, and an initially normal optic disc and fundus on ophthalmoscopy with the later development of optic disc pallor at 6-8 weeks, and no other abnormalities to explain the vision loss are suggestive of PION diagnosis [17]. Perioperative PION diagnosis is typically straightforward in that the vision loss is noticed soon after the patient wakes from a non-ocular surgical procedure [17].
Laboratory Studies
Differentiation between arteritic and non-arteritic PION is similar to the process of differentiation in AION. In patients over the age of 50 with arteritic PION, elevations of serum erythrocyte sedimentation rate (ESR) and C protein (CRP) are helpful in diagnosis. A serum CRP in conjunction with ESR has a sensitivity of up to 100% and specificity of 97% for GCA. Furthermore, inclusion of other acute phase reactants such as thrombocytosis (sensitivity = 57.0%, specificity = 96.5%), elevated white blood cell counts (sensitivity = 28.1%, specificity = 85.7%), and low hemoglobin (sensitivity = 46.3%, specificity = 92.9%) and hematocrit (sensitivity = 39.8%, specificity = 91.3%) can also indicate a possible diagnosis of GCA [18]. Non-arteritic PION does not lead to abnormal results for either test [17].
Imaging
Diffusion-weighted imaging (DWI) sequences on orbital and cranial MRI can show diffusion restriction (decreased apparent diffusion coefficient) in the posterior optic nerve [7]. In cases of acute ischemic injury to the posterior optic nerve, cytotoxic edema occurs and results in water molecules accumulating intracellularly from the extracellular space, restricting diffusion across the cell membrane [19]. Thus, ischemia of the pial branches supplying the periphery or of the central retinal artery supplying the center could both be theoretically determined using DWI, but only a few cases have reported diagnosing PION successfully in this manner [19].
Visual evoked potentials (VEP) may also be helpful in distinguishing PION from retrobulbar neuritis but is nonspecific [20]. In retrobulbar neuritis, there is a significant increase in the latent period for the major positive wave pattern VEP as well as a more prominent VEP latency change when compared to PION. This has been presumed to be due to unimpaired conductivity through the preserved axons in PION. VEP latency of 30 msec or more is usually indicative of retrobulbar neuritis rather than PION [20]. PION is more likely to cause a reduction in amplitude of VEP and is more likely to be permanent and irreversible changes as compared to optic neuritis. Note that in both retrobulbar neuritis and PION ancillary studies such as ERG and FA will also appear normal at time of presentation.[4]
Differential Diagnosis
The main differential diagnoses of PION include: retrobulbar neuritis, macular or retinal lesions, toxic agents, compression or inflammation of the posterior optic nerve [21]. These can be differentiated based on previously mentioned imaging techniques such as VEP, ERG, OCT, DWI MRI and others.
Treatment
Steroids
The protocol for steroid treatment for arteritic PION is an immediate introduction of intravenous and oral corticosteroids [17]. Intravenous steroid therapy could be administered as an emergency precaution followed by oral steroid taper. The exact dosage of oral steroid therapy will vary by patient, but the median initial dosage was 80mg/day of oral Prednisone. Steroid treatment should slowly be tapered after ESR and CRP level off at the lowest recorded measurement and are stable. Initial CRP stability followed by ESR stability historically occurs after 2 weeks of high dose oral steroids but can take longer. Treatment length is around 2.5 months total including time to taper the patient off steroids [1].
Similarly, studies involving steroid therapy for non-arteritic PION are a practice option. An 80mg initial dose of oral Prednisone given for 2 weeks and then tapered could be considered in non-arteritic PION but there are no randomized controlled clinical trial data to make an evidence-based recommendation for the treatment of non-arteritic PION [1].
Currently, there is no established treatment option for perioperative PION [3] and steroids have not been recommended typically for perioperative, non-arteritic PION. However, few case reports have suggested a combination of hyperbaric oxygen therapy and steroids could lead to success in treating perioperative PION when starting treatment within 72 hours of presentation[22]. General measures in perioperative PION include addressing the aforementioned risk factors, maintaining adequate mean arterial pressure during procedures including minimizing blood loss, minimizing prone positioning and maximizing venous outflow from the head intraoperatively.[4]
Practice Advisory for Spine Surgery
Preoperatively, assess for anemia, obesity, and vascular risk factors such as hypertension, diabetes, previous stroke, tobacco use, etc. High-risk patients must be informed that these conditions, along with prolonged procedures and considerable blood loss and hypotension, may increase risk of perioperative vision loss [23].
Intraoperatively, systemic blood pressure should be continuously monitored in high-risk patients and should be individually assessed for each patient based on their baseline blood pressure. Deliberate hypotension should be used only when it is absolutely essential, after multidisciplinary approach with anesthesiologist and surgeons. If deliberate hypotension is used, prolonged decreases in blood pressure must be treated accordingly. Hypertensive patients should have arterial pressure maintained at higher levels to prevent risks to end organs. Vasopressors for hypotension should be used only when deemed necessary on a case-by-case basis. Patients’ hemoglobin or hematocrit values should also be monitored often during surgery in high-risk patients with considerable blood loss. Transfusions may be used when necessary, and crystalloids or colloids can be used to maintain adequate blood volume [23]. Some positioning tips:[23]
- Position patient's head neutrally, forward, and level with or higher than the rest of the body. "Head holders" should only be used if surgeon considers head positioning too challenging without it.
- Avoid direct pressure on the eye to prevent retinal artery occlusion
- Check eye position periodically during surgery to identify any eye compression
- Staged spine are not recommended but may be used only if found situationally necessary
Postoperatively, vision must be checked when the patient awakens, and any visual concern requires immediate ophthalmologic consultation. Necessity of CT or MRI to assess causes of vision loss should be individually determined. Furthermore, adequate arterial oxygenation, hemodynamic status, and hemoglobin or hematocrit values should be maintained [23].
Course and Outcome
In arteritic PION, immediate steroid treatment may mitigate progression of vision loss or fellow eye involvement, but usually no significant improvement in an eye with existing vision loss [1]. In one study, steroid treatment for non-arteritic PION as compared to control groups with no treatment had significant improvements in visual field (P=0.030) and significant improvements in visual field (P<0.001) and visual acuity (P=0.031) compared to baseline as well [1]. Perioperative PION does not respond to steroid treatment, and vision loss is often bilateral, irreversible, and severe [17].
Additional Resources
- Porter D, Vemulakonda GA. Blood Pressure. American Academy of Ophthalmology. EyeSmart/Eye health. https://www.aao.org/eye-health/anatomy/blood-pressure-list. Accessed January 06, 2023.
References
- ↑ 1.00 1.01 1.02 1.03 1.04 1.05 1.06 1.07 1.08 1.09 1.10 1.11 1.12 1.13 1.14 1.15 1.16 1.17 1.18 1.19 1.20 Hayreh SS. Posterior ischaemic optic neuropathy: clinical features, pathogenesis and management. Eye. 2004;18(11):1188-1206. doi:10.1038/sj.eye.6701562.
- ↑ 2.0 2.1 2.2 Dunker S, Hsu HY, Sebag J, Sadun AA. Perioperative Risk Factors for Posterior Ischemic Optic Neuropathy. J Am Coll Surg. 2002;194(6):705-710.
- ↑ 3.0 3.1 3.2 3.3 3.4 3.5 3.6 Rucker JC, Biousse V, Newman NJ. Ischemic optic neuropathies. Curr Opin Neurol. 2004;17(1):27-35.
- ↑ 4.0 4.1 4.2 4.3 4.4 4.5 Wang MY, Brewer R, Sadun AA. Posterior ischemic optic neuropathy: Perioperative risk factors. Taiwan J Ophthalmol. 2020 Sep 11; 10(3):167-173. doi: 10.4103/tjo.tjo_41_20. PMID: 33110746; PMCID: PMC7585472.
- ↑ 5.0 5.1 5.2 Buono LM, Foroozan R. Perioperative posterior ischemic optic neuropathy: review of the literature. American Journal of Ophthalmology. 2005;139(5):956. doi:10.1016/j.ajo.2005.03.007.
- ↑ Oh DJ, Chhadva P, Kanu LN, Liu CY, Macintosh PW. Sudden-onset Blindness from a Spontaneous Carotid-cavernous Fistula with Secondary Central Retinal Artery Occlusion and Posterior Ischemic Optic Neuropathy. Neuro-Ophthalmology. 2018;43(2):107-113. doi:10.1080/01658107.2018.1488979.
- ↑ 7.0 7.1 Maramattom B, Sundar S, Thomas D, Panikar D. Postoperative posterior ischemic optic neuropathy (PION) following right pterional meningioma surgery. Annals of Indian Academy of Neurology. 2016;19(3):374-376. doi:10.4103/0972-2327.186826.
- ↑ 8.0 8.1 Cheng MA, Todorov A, Tempelhoff R, Mchugh T, Crowder CM, Lauryssen C. The Effect of Prone Positioning on Intraocular Pressure in Anesthetized Patients. Anesthesiology. 2001;95(6):1351-1355. doi:10.1097/00000542-200112000-00012.
- ↑ Epstein NE. Perioperative visual loss following prone spinal surgery: A review. Surg Neurol Int. 2016;7(Suppl 13):S347-60. Published 2016 May 17. doi:10.4103/2152-7806.182550
- ↑ Distefano AG, Pasol J. Posterior Ischemic Optic Neuropathy After Blepharoplasty. J Neuroophthalmol. 2018 Jun;38(2):200-201. doi:10.1097/WNO.0000000000000636. PMID: 29384801.
- ↑ Lin LY, Reshef ER, Lansberg MP, et al. Posterior Ischemic Optic Neuropathy in the Setting of Cocaine-Induced Orbital and Sinonasal Inflammation [published online ahead of print, 2022 Apr 25]. Ophthalmic Plast Reconstr Surg. 2022;10.1097/IOP.0000000000002181. doi:10.1097/IOP.0000000000002181
- ↑ Huang S, Curragh D, Selva D, Davis G. Posterior Ischemic Optic Neuropathy Following Contralateral Endoscopic Orbital Decompression. Ophthalmic Plast Reconstr Surg. 2018 Nov/Dec;34(6):598-599. doi: 10.1097/IOP.0000000000001214. PMID: 30418396.
- ↑ Wang MY, Brewer R, Sadun AA. Posterior ischemic optic neuropathy: Perioperative risk factors. Taiwan J Ophthalmol. 2020;10(3):167-173. Published 2020 Sep 11. doi:10.4103/tjo.tjo_41_20
- ↑ Tauscher RG, Bison HS, Simon SS, Volpe NJ. Intracranial Posterior Ischemic Optic Neuropathy and Ophthalmic Artery Occlusion. J Neuroophthalmol. 2022 Jun 1;42(2):e476-e478. doi: 10.1097/WNO.0000000000001313. Epub 2021 Jul 23. PMID: 34310459.
- ↑ Keller B, Rauf Y, Hinduja A. Posterior Ischemic Optic Neuropathy, a previously unknown complication of lung transplantation. Neurology. May 2019.
- ↑ Selvaraj V, Sacchetti D, Finn A & Afriyie KD. (2020) Acute Vision Loss in a Patient with COVID-19. J Infect Dis Res, 3(S2): 14.
- ↑ 17.0 17.1 17.2 17.3 17.4 17.5 17.6 Hayreh SS. Ischemic optic neuropathy. Progress in Retinal and Eye Research.2009;28(1):34-62.
- ↑ Costello F, Zimmerman M, Podhajsky P, Hayreh S. Role of Thrombocytosis in Diagnosis of Giant Cell Arteritis and Differentiation of arteritic from Non-Arteritic Anterior Ischemic Optic Neuropathy. European Journal of Ophthalmology. 2004;14(3):245-257. doi:10.1177/112067210401400310.
- ↑ 19.0 19.1 Bhatt NP, Morales RE, Mathews MK. MRI findings in Post-operative Bilateral Posterior Ischemic Optic Neuropathy. Open J Ophthalmol. 2013;3:51-53.
- ↑ 20.0 20.1 Veselinovic D, Duric S. Differentiation of posterior ischemic optic neuropathy from retrobulbar neuritis with pattern evoked visual potential response. Facta Universitatis. 2004;11(3):127-130.
- ↑ Boor K, Kovacs K, Rozsa A, Panczel G, Szilvassy I, Gacs G. Posterior ischemic optic neuropathy. Ideggyogy Sz. 2009;62(5):191-194.
- ↑ Allashem HM, Sward DG, Sethuraman K, Matthews MK. Hyperbaric oxygen therapy for perioperative posterior ischemic optic neuropathy: a case report. Undersea & Hyperbaric Medicine : Journal of the Undersea and Hyperbaric Medical Society, Inc. 2019 Sep - Dec - Fourth Quarter;46(5):701-707.
- ↑ 23.0 23.1 23.2 23.3 Practice Advisory for Perioperative Visual Loss Associated with Spine Surgery 2019. Anesthesiology. 2018;130(1). doi:10.1097/ALN.0000000000002503.