Port Delivery System
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
Video summarizing the major steps of the surgical implantation of the PDS, including peritomy, scleral dissection, pars plana laser ablation, pars plana incision, implant insertion, and closure of the conjunctiva and Tenon's capsule.
Video depicting insertion of the refill-exchange needle through the septum of the PDS implant, followed by refill-exchange (simultaneous extraction of implant contents into the needle tip and replacement with fresh drug).
Background & History
Intravitreal injection is a technique that allows for concentrated, localized drug delivery to the posterior segment while minimizing systemic adverse effects.[1] The procedure is used to administer a variety of drugs, including but not limited to antimicrobial, anti-inflammatory, and anti-VEGF agents. Intravitreal injection of anti-VEGF medications in particular has redefined the treatment landscape for neovascular diseases such as neovascular (wet) age-related macular degeneration (nAMD) and proliferative diabetic retinopathy, two of the leading causes of blindness in adults over the age of 40. AMD specifically is the leading cause of vision loss and blindness for Americans aged 65 and older. The ANCHOR and MARINA studies demonstrated that ranibizumab was highly effective in halting progression and even reversing visual impairment from nAMD.[2][3] The READ2, RESOLVE, RESTORE, and RISE/RIDE studies revealed similar findings for ranibizumab in treating diabetic macular edema.[4] Regular injection of ranibizumab or other anti-VEGF medications such as aflibercept or bevacizumab is now considered the gold standard for treatment of nAMD and an effective option for center-involved diabetic macular edema.[5][6]
However, intravitreal injection is not without risk. Most adverse effects are mild, such as local discomfort, subconjunctival hemorrhage, and temporary ocular hypertension, but can be as severe as endophthalmitis.[1] Furthermore, the short half-life of anti-VEGF agents necessitates frequent injections indefinitely, with most regimens beginning with monthly administration. This need for many regular visits increases the cumulative risk for a complication and places extra financial, time, and emotional burden on patients.[7] Longitudinal studies on nAMD patients who received anti-VEGF injections as part of clinical trials have revealed that a portion of patients lose gains in visual acuity 5-7 years after exiting the study;[8][9] the burden of repeated injections may decrease adherence to the strict treatment regimen and exacerbate this backsliding.[10] In the Preferences and Trends surveys from the American Society of Retina Specialists, US retina physicians consistently listed reduced treatment burden and long acting/sustained delivery as the 2 greatest unmet needs in the treatment of their patients with nAMD, with as many as 75.2% and 66.2% of respondents agreeing with the respective statements from 2018 to 2021.[11][12][13]
One potential solution to reduce the need for regular injections is the Port Delivery System (PDS or Susvimo, commercial [Genentech, Inc., San Francisco, CA]) ocular implant, a small, refillable device designed for permanent implantation within the sclera to allow for continuous passive diffusion of drug into the vitreous cavity. By continuously delivering medication over extended period of time, the PDS aims to maintain the clinical benefits of monthly intravitreal anti-VEGF therapy while decreasing the injection burden to only the refill-exchange procedure, in which the device’s medication stores are replenished. The PDS was originally designed for specially formulated 100 mg/mL ranibizumab and is FDA-approved for the treatment of nAMD, although other drugs for delivery are in clinical trials.
As of October 2022, Genentech has issued a recall of the Susvimo device and related insertion instruments due to reported cases of septum dislodgement.[14] New implantations are currently paused, although refill-exchange procedures for previously implanted devices continue.
Design
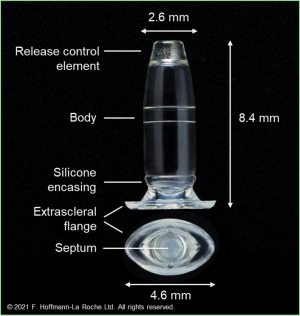
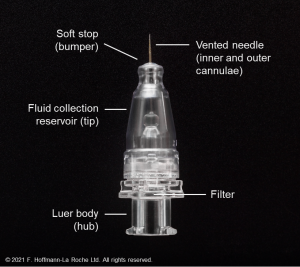
This novel drug-delivery platform is comprised of four components contained in a silicone encasement.
- Body: A hollow, refillable reservoir in which up to 0.02 mL of medication can be stored
- Release control element: A titanium medium between the body of the device and the vitreous that regulates the rate of passive diffusion of ranibizumab into the vitreous
- Septum: A self-sealing interface on the extrascleral surface of the device that allows for repeated filling of the device with ranibizumab via needle injection
- Extrascleral flange: A flaring of the silicone encasement designed to sit superficial to the sclera and anchor the device deep to the conjunctiva without the need for suture
The device is 8.4 mm in length and 4.6 mm in diameter at the flange, tapering to 2.6 mm at the release control element at the interface with the vitreous. Following implantation, the majority of the device is located in the vitreous, with a small segment traversing the sclera and widening into the flange to allow for passage of a needle through the septum and into the body during refilling.
Patient Selection
Indications
The PDS was approved by the FDA in 2021 as the commercial Susvimo implant for the treatment of nAMD in patients who have previously shown response to two or more intravitreal VEGF inhibitor injections.[15]
Indications for Susvimo may widen in the future as clinical trials studying its use in other ocular disease entities and other medications are ongoing. The phase 3 PAGODA and PAVILION trials are currently underway to investigate the efficacy of Susvimo in treating diabetic macular edema and diabetic retinopathy, respectively.[16][17] Although new implantations are temporarily suspended, trials are expected to resume by end of 2023; see “Septum Dislodgment” in the Complications section and the Manufacturer Recall section below.
Contraindications
Absolute contraindications to Susvimo include the following.[15]
- Ocular and periocular infections
- Active intraocular inflammation
- Hypersensitivity to ranibizumab or other inactive components of Susvimo
Relative contraindications & Preoperative evaluation
Assessment of the proper patient to receive the Susvimo implant is important. The retinal surgeon should consider patients who have shown adequate response to prior anti-VEGF injection therapy, shown a need for ongoing medication dependence to control the retinal disease, and are motivated to reduce the burden of regular-interval frequent intravitreal injections.
The retinal doctor should also carefully consider ocular risk factors that may be relative contraindications to implantation of Susvimo, such as conjunctival scarring or surgery, thinning of the conjunctival tissue, glaucoma, or keratoconjunctivitis sicca (excessive dry eye surface). Additionally, some systemic factors should be considered as potential risks for conjunctival erosion or retraction. For instance, patients with rheumatoid arthritis, lupus, granulomatosis with polyangiitis, floppy eyelid syndrome, or frequent rubbing or “knuckling” of the eye may not be good candidates for a permanent intraocular implant.
Antithrombotic medications such as anticoagulants and NSAIDs should be held prior to the procedure to reduce risk of intraoperative bleeding and postoperative complications such as vitreous hemorrhage.
Surgical Technique
Initial Implantation
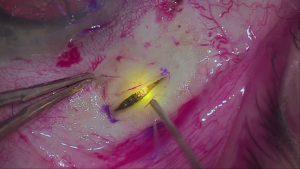
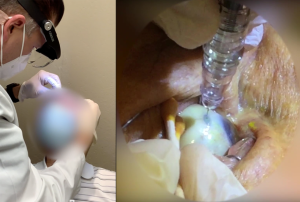
Insertion of the Susvimo implant involves exposure and dissection of the sclera, allowing for insertion of the device into the vitreous, followed by careful closure to avoid post-surgical complications. the procedure can be summarized in seven major steps as follows.[15]
0. Preparation: Prepare the patient with induction of local anesthesia, standard surgical sterile procedures, dilation of the pupil, placement of a speculum, and insertion of an infusion line, ideally in the inferotemporal quadrant.
1. Peritomy: After identifying the device insertion site 4 mm posterior to the limbus in the superotemporal quadrant, placing a traction suture is recommended to improve exposure for the remainder of the procedure. Perform a 6 mm by 6 mm limbal peritomy of both the conjunctiva and Tenon’s capsule with nontoothed forceps to preserve tissue integrity and allow for a bilayer closure. Generous blunt dissection is optimal to allow for adequate tissue coverage of the flange with minimal tension following implantation. Hemostasis is particularly important in this step and in stages 3, 4, and 5.
2. Implant preparation: Draw the ranibizumab into the syringe using the filter needle, then switch to the fill needle and remove any air bubbles. Using the included insertion tool carrier, advance the syringe needle through the septum of the implant. Under the microscope, fill the implant slowly, over 5-10 seconds, to avoid injection of air into the body reservoir. When the implant is full as evidenced by a dome of fluid at the tip of the release control element, remove the syringe from the insertion tool carrier and replace it with the insertion tool handle.
3. Scleral dissection: After ensuring the scleral surface is dry, use an MVR blade to dissect through the sclera to the pars plana at the insertion site, creating an incision precisely 3.5mm wide with squared edges to avoid tearing of tissue during device insertion. Incisions greater than 3.5 mm must be corrected by suturing wound edges before implantation.
4. Pars plana laser ablation: Ablate the visible choroid in all exposed pars plana tissue with a 532 nm laser to prevent bleeding during implantation, paying close attention to the corners. Laser should start at 300 mW and 1000 ms and be applied as contiguous, overlapping single spots, but not painting strokes. As many as 70-100 laser spots may be executed before observing the desired endpoints of graying color, perforated appearance, and/or vitreous seeping through the pars plana. As this procedure may enlarge the incision, confirm the incision length of precisely 3.5 mm after this step before proceeding.
5. Pars plana incision: Open the pars plana by passing a 3.2 mm slit knife through the center of the scleral incision, taking care to avoid lateral movements which may enlarge the incision or induce bleeding. Prolapse of vitreous during this step or the following step may be cleaned using a vitrector at the surgeon’s discretion; do not use other instruments such as sponges to avoid vitreous traction.
6. Implant insertion: Remove the insertion tool handle loaded with the implant from the insertion tool carrier, being sure not to touch the implant to any surface to avoid placing an intraoacular foreign body. Stabilize the globe and use the insertion tool handle to insert the implant into the insertion site perpendicularly, aligning the long axis of the flange with the wound. A twisting motion may be used to pass the device through the incision, and the infusion line can be activated for counter pressure. Continue pushing the implant into the globe until the gripper tips that grasp the device press firmly against the sclera.
7. Conjunctival and Tenon’s closure: Close both layers over the implant using interrupted 8-0 or 7-0 Vicryl or gut sutures, ensuring scleral bites at the limbus such that both layers overlap the peripheral cornea. Remove infusion cannula and traction suture, correcting any persistent leaks with suture as needed. Finally, check IOP digitally and implant placement with indirect ophthalmoscopy to confirm proper positioning.
Refill-Exchange Procedure
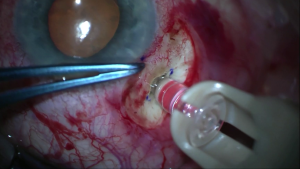
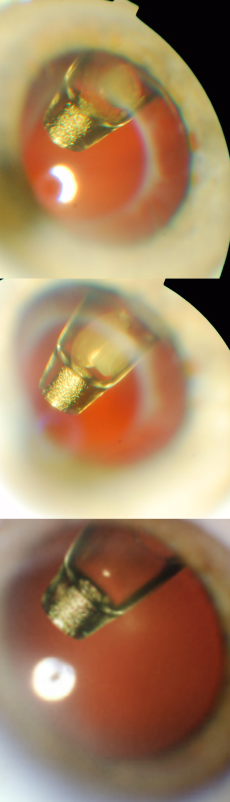
The PDS implant is refilled during a minimally invasive in-clinic refill-exchange procedure using the specially designed PDS refill needle, via the self-sealing septum.[15] The refill needle is a 34G double cannula with a vented needle and fluid collection reservoir that enables exchange of the implant contents with fresh ranibizumab 100 mg/mL. One 100-µL refill-exchange stroke can remove over 98% of the implant contents and replace it with fresh drug. Previous contents are exchanged fresh medication in a single stroke, with no significant bolus during the procedure.
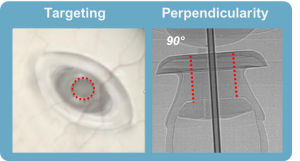
The innovative refill-exchange procedure should be approached differently than an intravitreal injection as it requires precise targeting and a strictly perpendicular approach. The refill-exchange procedure should be performed under strict aseptic conditions with optimization of visualization, using magnification and task lighting to set the stage for refill-exchange success.
Of note, if there is any significant resistance encountered, withdraw the refill needle. Increased pressure will not overcome imprecise alignment. Do not tilt or twist if there is resistance, as it may bend the needle or cause damage to septum or overlying tissue. Reorient and insert again, taking care to ascertain perpendicularity, alignment, and good visualization.
The refill needle achieves a “soft stop” which must remain in contact with the conjunctiva throughout the refill-exchange procedure, which should be done slowly over ~5–10 seconds. Use of a cotton-tipped applicator also helps to stabilize the globe and minimize eye movement. Precise targeting to the septum center with a perpendicular approach is key for success.
The PDS refill-exchange procedure differs from intravitreal injection and requires a unique and precise skillset. The experiences during refill-exchange in clinical trials have informed the evolution and refinement of PDS procedures. Adherence to the refill-exchange procedure methodology maximizes optimal outcomes.
Outcomes
The PDS remains a novel device at present and thus has limited research regarding long-term outcomes. The flagship phase 3 clinical trial that validated Susvimo and laid the foundation for its FDA approval was the Archway study, in which 418 patients were separated into either the PDS group or intravitreal ranibizumab group.[18] The trial showed that over 40 weeks, 100 mg/mL ranibizumab administered via PDS with refill-exchange every 24 weeks was noninferior to and equally efficacious as 0.5 mg ranibizumab injections every 4 weeks. Patients in the PDS group were eligible for supplemental intravitreal 0.5 mg ranibizumab prior to refill-exchange if monthly routine study visits demonstrated worsening disease, however 98.4% of patients did not require rescue injection. In addition to proving medically efficacious, 93% of patients in the PDS group reported preference of the PDS over standard intravitreal injections, demonstrating at least partial reduction in the burden of nAMD treatment.
The currently ongoing Portal extension trial will provide more long-term data regarding efficacy and safety of Susvimo. Subgroup analysis of patients from the phase 2 Ladder trial who have had the PDS for 5 or more years promisingly demonstrated visual and anatomical stability from the Ladder baseline visit.[19]
Complications
Complications and adverse events during and after Susvimo implantations were uncommon in study and include conjunctival erosion, retraction, bleb, vitreous hemorrhage, endophthalmitis, among others. Of these, many have been associated with inadequate management of the conjunctiva or Tenon’s capsule during the procedure. These associations highlight the need for careful intraoperative handling and closure of both the conjunctiva and Tenon’s capsule. Updated versions of protocols for clinical trials and increased surgical experience with the device has resulted in a decrease in all complications over time.
- Endophthalmitis: In the original wet macular degeneration studies, Susvimo implantation was associated with a 3-fold higher rate of endophthalmitis (infection in the eye) than monthly intravitreal injections of ranibizumab (in clinical trials, 2.0% of implant eyes vs. 0.5% of control eyes receiving intravitreal ranibizumab injections). Most of these cases are associated with conjunctival retraction or erosion, and improved implantation protocol addressing this risk has reduced the incidence of endophthalmitis. Recently published multi-year data of the implant in diabetic patients (381 patients in PAGODA and 106 patients in PAVILION trials), for example, showed zero cases of endophthalmitis.
- Vitreous Hemorrhage: Prior to updating methodology to include laser ablation of the pars plana in the phase 2 Ladder trial, incidence of vitreous hemorrhage during device implantation was as high as 50%. More recent clinical trial data indicates a rate of vitreous hemorrhage between 5 and 10% . Adequate laser ablation of the choroid at the pars plana helps to mitigate this risk. The majority of recorded cases of vitreous hemorrhage were mild and resolved spontaneously, but vitrectomy may be required if the blood does not clear.
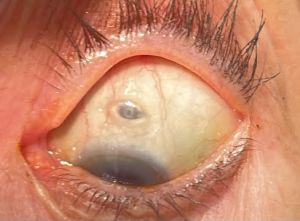
- Conjunctival erosion or retraction: Full thickness erosion of the conjunctiva at the device or retraction of the conjunctiva at the limbus or radial peritomy have been observed and are associated with a heightened risk of endophthalmitis.
- Conjunctival Bleb: Conjunctival thickening or accumulation of fluid deep to the conjunctiva may develop superficial to the device, possibly obscuring the septum and complicating the refill-exchange procedure.
- Retinal Detachment: Meticulous examination for present or impending retinal detachments or breaks should be performed prior to device insertion. Abnormalities detected prior to or after implantation should be treated as indicated and refills should be held until they are repaired.
- Implant Dislocation: In very rare cases, the device has been reported to shift further into or out of the vitreous cavity, especially if the scleral incision was made too long during the initial incision or after laser ablation of the pars plana.
- Postoperative Reduced Visual Acuity: In clinical trials, mean VA fell by 4 letters in the first month following implantation, then recovered to 2 letters in the following month.
- Septum Dislodgement: Susvimo devices manufactured in the second batch of produced implants have shown possible dislodgement of the septum at a rate of 2.3% during implantation and 0.63% during refill-exchange procedures. This phenomenon has not been reported in devices produced for ongoing clinical trials (PAGODA, PAVILION, or Portal). See the Manufacturer Recall section below.
Manufacturer Recall
In October 2022, Susvimo manufacturer Genentech issued a voluntary recall of the Susvimo Ocular Implant and Insertion Tool Assembly due to reports of the septum of the device dislodging from the flange and into the body of the device in clinical trials.[14] As of August 31st, 2022, there rate of septum dislodgement was 2.3% during implantation and 0.63% during refill-exchange procedures. No cases have been reported in Phase 2 or commercial implants, however additional testing of the commercial supply revealed an unacceptably high rate of dislodgement, triggering the recall. The manufacturing has since been under further evaluation, testing, and refinement. It is anticipated that ongoing clinical trials of the PDS will be resumed near the end of 2023 and commercial availability in early 2024 will be pursued.
In addition to pausing new implantations, Genentech recommends against performing refill-exchange in patients whose devices have had septum dislodgement; whether or not to remove the device should be informed by shared decision-making by the patient and physician. In patients with a normally functioning implant, Genentech recommends continuing refill-exchange procedures and has left Susvimo refill vials and refill needles available.
To date, no further information about the current state of the device nor improvements to resolve the septum dislodgement issue are available.
References
- ↑ Jump up to: 1.0 1.1 Peyman GA, Lad EM, Moshfeghi DM. Intravitreal injection of therapeutic agents. Retina. Jul-Aug 2009;29(7):875-912. doi:10.1097/IAE.0b013e3181a94f01
- ↑ Brown DM, Kaiser PK, Michels M, et al. Ranibizumab versus verteporfin for neovascular age-related macular degeneration. The New England journal of medicine. Oct 5 2006;355(14):1432-44. doi:10.1056/NEJMoa062655
- ↑ Rosenfeld PJ, Brown DM, Heier JS, et al. Ranibizumab for neovascular age-related macular degeneration. The New England journal of medicine. Oct 5 2006;355(14):1419-31. doi:10.1056/NEJMoa054481
- ↑ Thomas BJ, Shienbaum G, Boyer DS, Flynn HW, Jr. Evolving strategies in the management of diabetic macular edema: clinical trials and current management. Can J Ophthalmol. Feb 2013;48(1):22-30. doi:10.1016/j.jcjo.2012.11.012
- ↑ Flaxel CJ, Adelman RA, Bailey ST, et al. Age-Related Macular Degeneration Preferred Practice Pattern(R). Ophthalmology. Jan 2020;127(1):P1-P65. doi:10.1016/j.ophtha.2019.09.024
- ↑ Flaxel CJ, Adelman RA, Bailey ST, et al. Diabetic Retinopathy Preferred Practice Pattern(R). Ophthalmology. Jan 2020;127(1):P66-P145. doi:10.1016/j.ophtha.2019.09.025
- ↑ Senra H, Balaskas K, Mahmoodi N, Aslam T. Experience of Anti-VEGF Treatment and Clinical Levels of Depression and Anxiety in Patients With Wet Age-Related Macular Degeneration. Am J Ophthalmol. May 2017;177:213-224. doi:10.1016/j.ajo.2017.03.005
- ↑ Rofagha S, Bhisitkul RB, Boyer DS, Sadda SR, Zhang K, Group S-US. Seven-year outcomes in ranibizumab-treated patients in ANCHOR, MARINA, and HORIZON: a multicenter cohort study (SEVEN-UP). Ophthalmology. Nov 2013;120(11):2292-9. doi:10.1016/j.ophtha.2013.03.046
- ↑ Comparison of Age-related Macular Degeneration Treatments Trials Research G, Maguire MG, Martin DF, et al. Five-Year Outcomes with Anti-Vascular Endothelial Growth Factor Treatment of Neovascular Age-Related Macular Degeneration: The Comparison of Age-Related Macular Degeneration Treatments Trials. Ophthalmology. Aug 2016;123(8):1751-1761. doi:10.1016/j.ophtha.2016.03.045
- ↑ Sharma A, Kumar N, Parachuri N, Kuppermann BD, Bandello F, Regillo CD. Ranibizumab port delivery system (RPDS): realising long awaited dream of prolonged VEGF suppression. Eye (Lond). Mar 2020;34(3):422-423. doi:10.1038/s41433-019-0479-y
- ↑ Stone TW. ASRS 2018 Preferences and Trends Membership Survey. presented at: American Society of Retina Specialists Annual Meeting; 2018; Chicago, IL.
- ↑ Hahn P, Stone TW. ASRS 2020 Preferences and Trends Membership Survey. presented at: American Society of Retina Specialists Annual Meeting; 2020; Chicago, IL.
- ↑ Hahn P. ASRS 2021 Preferences and Trends Membership Survey. presented at: American Society of Retina Specialists Annual Meeting; 2021; Chicago, IL.
- ↑ Jump up to: 14.0 14.1 Subject: Voluntary recall of the SUSVIMOTM Ocular Implant. Genentech; 2022. Accessed 08/29/2023. https://www.gene.com/download/pdf/Susvimo_DHCP_Important_Prescribing_Information_2022-10-18.pdf
- ↑ Jump up to: 15.0 15.1 15.2 15.3 Susvimo. Package Insert. Genentech; 2022. Accessed 08/29/2023. https://www.gene.com/download/pdf/susvimo_prescribing.pdf
- ↑ Hoffmann-La Roche. This Study Will Evaluate the Efficacy, Safety, and Pharmacokinetics of the Port Delivery System With Ranibizumab in Participants With Diabetic Macular Edema Compared With Intravitreal Ranibizumab (Pagoda). ClinicalTrials.gov identifier: NCT04108156. Updated July 14, 2023. Accessed August 29, 2023. https://clinicaltrials.gov/study/NCT04108156
- ↑ Hoffmann-La Roche. A Multicenter, Randomized Study in Participants With Diabetic Retinopathy Without Center-involved Diabetic Macular Edema To Evaluate the Efficacy, Safety, and Pharmacokinetics of Ranibizumab Delivered Via the Port Delivery System Relative to the Comparator Arm (PAVILION). ClinicalTrials.gov identifier: NCT04503551. Updated August 25, 2023. Accessed August 29, 2023. https://clinicaltrials.gov/study/NCT04503551
- ↑ Holekamp NM, Campochiaro PA, Chang MA, et al. Archway Randomized Phase 3 Trial of the Port Delivery System with Ranibizumab for Neovascular Age-Related Macular Degeneration. Ophthalmology. Mar 2022;129(3):295-307. doi:10.1016/j.ophtha.2021.09.016
- ↑ Howard J, Cordeiro MC, Singh N, Gune S. Long-term efficacy and safety of the Port Delivery System with ranibizumab in patients with neovascular age-related macular degeneration: Results from the Portal 5-year subgroup analysis. presented at: 2023 ARVO Annual Meeting; 2023; New Orleans, LA.