Pentosan Polysulfate Maculopathy
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
Pentosan polysulfate sodium (PPS) is the only Food and Drug Administration, USA (FDA) approved oral medication for the treatment of interstitial cystitis (IC). It has been used extensively to treat IC since its approval in 1996. Several recent studies have described a unique, progressive maculopathy associated in a dose-dependent manner with long term use of the medication. It often causes symptoms of prolonged dark adaptation and nyctalopia and may cause blurred vision. While visual acuity may initially remain intact, cystoid macular edema, macular neovascularization, and retinal pigment epithelium (RPE) atrophy may occur resulting in severe vision loss. Although its pathogenesis has not yet been definitively established, clinical imaging suggests that PPS maculopathy primarily impacts the RPE and the RPE-photoreceptor interface and results in a characteristic pattern of retinal imaging findings.
Disease Entity
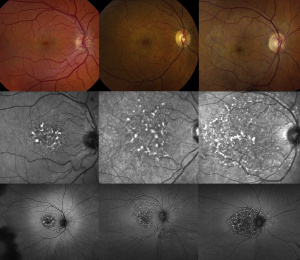
Long-term use of pentosan polysulfate sodium (PPS) is strongly linked to a vision-threatening macular disease characterized by a unique constellation of retinal imaging findings.
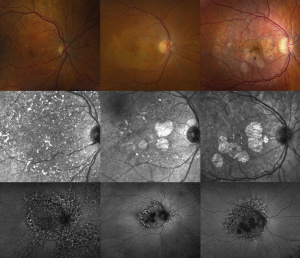
Disease
Pentosan polysulfate sodium (trade name Elmiron, Janssen Pharmaceuticals, Titusville, NJ) is a semisynthetic sulfated polysaccharide that is the only FDA-approved oral therapy for interstitial cystitis (IC). Interstitial cystitis, or bladder pain syndrome, is a syndrome of urinary frequency, urgency, and chronic pelvic discomfort, without an otherwise identifiable cause. It is much more common in women and is estimated to impact over one million people in the United States.[1]
Pentosan polysulfate has been prescribed widely for IC in the US since its FDA approval in 1996, and was used for many years prior in an off-label fashion.[2] The American Urological Association currently recommends PPS as a second line therapy for IC after the failure of conservative management, and numerous international bodies recommend it as a first- or second-line agent.[1][3][4][5] Pentosan polysulfate is thought to protect the bladder lining from irritants by replacing disrupted glycosaminoglycans along the bladder urothelium.[4][6] A 2018 report first described a unique macular disease associated with long-term use of PPS in six patients.[7] Numerous subsequent studies have further characterized the disease and strengthened its dose-dependent association with PPS use.[8][9][10] The totality of the evidence across many studies is strongly suggestive of a causal relationship between PPS use and this maculopathy.
Affected patients commonly experience difficulty reading, prolonged dark adaptation, and in some cases loss of visual acuity.[7][11][12] Retinal imaging findings suggest a primary insult to the retinal pigment epithelium (RPE), RPE-photoreceptor interface, and/or choriocapillaris.[7][11][12][13][14] Although most cases manifest disease in the posterior pole, some eyes have involvement of the far peripheral retina.[11][12] Pentosan polysulfate maculopathy may result in RPE and outer retinal atrophy, cystoid macular edema (CME), vitelliform maculopathy, and macular neovascularization (MNV).[11][12][15][16]
Risk Factors
The primary risk factor for development of PPS maculopathy is high cumulative PPS exposure.[12] Numerous studies have suggested a dose-response relationship between the characteristic maculopathy and PPS exposure.[4][10][12][17][18][19][20] One study demonstrated PPS maculopathy prevalence rates of 12.7%, 30.0%, and 41.7% among cumulative exposure groups of 500—999 g, 1000-1500 g, and >1500 g, respectively.[10]
As of December 2020, there is one documented case of PPS-associated maculopathy in a patient with three years of exposure and a cumulative dose of 0.33 kg. and only four cases reported in patients with less than 0.65 kg cumulative exposure, equivalent to six years at the standard daily dose of 300 mg.[21] However, as screening programs become more widely implemented, it is likely that cases of early PPS maculopathy will be identified at lower exposures.
A study that broadly evaluated medical comorbidities and medications among patients with IC found that aside from PPS exposure, no covariate was associated with the presence of the characteristic maculopathy.[8] Although the medication is metabolized and excreted by the liver, spleen, and kidney, no links have yet been found to hepatic, renal, or splenic dysfunction.[4][11] No genetic associations have been found in analyses for genes linked to retinal disease.[7][11][12]
General Pathology
Multimodal retinal imaging indicates that PPS maculopathy is caused by a primary abnormality at the RPE, RPE-photoreceptor interface, and/or choriocapillaris.[7][11][12] Characteristic findings in early disease include darkly pigmented nodular excrescences at the level of the RPE amidst yellowish subretinal deposits.[7][11] Disruption to choriocapillaris flow may also be seen before the development of clinically apparent macular toxicity in some eyes.[13][14] The pathology typically involves and centers around the fovea and affects both eyes symmetrically.[7][11][12] Cystoid macular edema, MNV, and RPE atrophy occur in some cases and can result in severe visual acuity loss. RPE atrophy appears to start in a parafoveal multifocal pattern and coalesce to involve the foveal center in severe cases.[11]
Pathophysiology
The pathophysiology of PPS maculopathy is not yet well understood. Pentosan polysulfate has wide-ranging biological activities including anticoagulant, anti-inflammatory, and fibrinolytic properties.[4] The backbone of the PPS molecule is similar to a glycosaminoglycan, which have myriad physiologic effects. Pentosan polysulfate also has a high negative charge which may lead to nonspecific interactions with positively charged molecules. A strong dose-dependent association is reported in the literature between PPS exposure and a progressive maculopathy that continues even after the drug is discontinued.[22]
It is speculated that PPS causes a disruption in RPE-photoreceptor homeostasis leading to misprocessing of photoreceptor outer segments. The interphotoreceptor matrix, which supports the photoreceptor-RPE interface, is made up of glycosaminoglycans which PPS could disrupt owing to its similar structure.[23][24] [25]Alternatively, some believe that long-term inhibition of fibroblast growth factor signaling may be responsible for PPS maculopathy. In vitro studies have found that PPS inhibits fibroblast growth factors that are an important part of retinal health maintenance.[26][27] Finally, OCT angiography studies suggesting early choriocapillaris injury may point to other mechanisms of injury.
Murine research has demonstrated significant retinal functional deficits on electroretinography in mice with primary RPE toxicity that have been exposed to high levels of PPS.[28] [29]Additional research is required to make definitive conclusions regarding the disease pathogenesis.
Primary Prevention
Avoidance of PPS therapy appears to be the only method for primary prevention of PPS maculopathy. However, IC can also carry a high symptom burden and some patients report significant benefit from PPS use. Prescribers should consider counseling patients regarding the risk of vision loss associated with PPS so that they can make an informed decision regarding using the medication. If they choose to take PPS, providers should prescribe the minimum effective dose and duration of treatment.[30]
Diagnosis
While history and dilated fundus examination (DFE) can be suggestive of PPS maculopathy, multimodal retinal imaging, particularly fundus autofluorescence imaging, is helpful. In some cases, the clinician must integrate findings from the examination, fundus autofluorescence imaging, near infrared reflectance imaging, and optical coherence tomography to establish the diagnosis.
Screening
Ophthalmologists at the Emory Eye Center, where this condition was initially described, monitor patients taking PPS or diagnosed with PPS maculopathy annually with a comprehensive retinal evaluation including color fundus photography, FAF, OCT, and NIR imaging. Exams are continued for the duration of treatment or beyond if there is suspicion for potential maculopathy development. A study by The Macula Society suggested a similar screening approach.[21] Most documented cases have occurred after 3 years of use or longer.[4] A drug labeling change issued in 2020 includes a warning about the increased risk for retinal pigmentary changes and recommends a detailed ophthalmologic evaluation prior to starting treatment.[4]
Another group has recommend a baseline screening exam within 6 months of starting the drug and then annually as patients approach 500 g of cumulative exposure (approximately 4.6 years on the standard 300 mg daily dose).[12] Patients with low
History
Providers should ask patients with suspected PPS maculopathy about the daily dose and duration of PPS intake. It may also be useful to ask about history of or ongoing tobacco use, although no such link has been established to date.
To gauge the functional impact of PPS maculopathy, it is useful to ask patients about difficulty adjusting to dim light, difficulty seeing at night, blurred vision, metamorphopsia, and difficulty reading. Pentosan polysulfate maculopathy has also been identified in patients who are not reporting any visual symptoms.[11][12] Thus, a negative history for visual symptoms does not rule out a mild manifestation of the disease.
In order to frame discussions regarding PPS cessation, it is helpful to inquire about IC disease status. Last, it is helpful to obtain contact information for the PPS prescriber to facilitate discussions regarding discontinuation of PPS therapy.
Physical examination
Although exam findings may be more subtle than those on fundus imaging, comprehensive evaluation with DFE should be performed for a patient suspected of having PPS maculopathy.
Signs
Examination may reveal hyperpigmented macular spots and yellowish subretinal deposits in early disease, and patchy parafoveal RPE atrophy, or more widespread atrophy, in severe disease.[7][11][12][31] Some eyes may manifest cystoid macular edema or CNV.[11][15][16] A normal DFE does not rule out PPS maculopathy since patients often have manifestations of disease on imaging that are not readily apparent on examination.
Symptoms
Common symptoms of PPS maculopathy include prolonged dark adaptation, nyctalopia, and difficulty reading or blurred vision, often in the setting of normal BCVA.[7][11][12][15][16] Scotoma in the setting of RPE atrophy, and distorted vision have also been reported. [11][12][16][31] Severe vision loss can occur in the setting of CME, MNV, and/or RPE atrophy.[10][11][31] A comprehensive study of visual function in PPS maculopathy demonstrated Low Luminance Questionnaire scores to be among the lowest ever reported with this tool.[32]
Clinical diagnosis
Clinical diagnosis may be highly suggested in patients with characteristic symptoms, DFE, and history of extensive PPS use. However, multimodal retinal imaging is typically necessary to definitively diagnose PPS maculopathy.
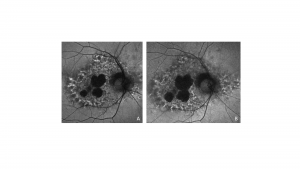
Characteristic features of PPS maculopathy were defined in one study as the following: “(1) bilateral pathology centered on the fovea; (2) fundus photography revealing paracentral macular hyperpigmented spots, pale yellow deposits, and/or patchy retinal pigment epithelium atrophy; (3) a dense array of hyper- and hypoautofluorescent spots and reticular fundus autofluorescence imaging abnormalities; and (4) foci of nodular retinal pigment epithelium enlargement on OCT imaging corresponding to hyperreflectance on near infrared reflectance imaging.”[8]
Diagnostic procedures
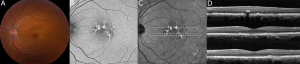
Fundus photography, fundus autofluorescence imaging (FAF), optical coherence tomography (OCT), and near-infrared reflectance imaging (NIR) are useful imaging modalities to establish a diagnosis of PPS maculopathy. Imaging findings are typically symmetric between both eyes, with rare cases of disease asymmetry.[11][12][21]
Color fundus photography typically shows more subtle manifestations compared to FAF. Hyperpigmented macular spots and deep yellowish subretinal deposits may be apparent, particularly in milder cases.[11][12][15][16] Patchy parafoveal RPE atrophy manifests in more advanced cases.[7][11][12][31]
Fundus autofluorescence imaging reveals a striking, densely packed array of hyper- and hypoautofluorescent spots typically centered on and involving the fovea.[4][7][11][12] Hyperautofluorescent spots colocalize with pigmented spots and yellow subretinal deposits apparent on color fundus photography.[7][11][12] In cases where the disease extends to the periapillary region, there is typically a hypoautofluorescent peripapillary halo.[11][12] RPE atrophy may also be noted in more severe cases, initially as multifocal parafoveal lesions, that ultimately coalesce and encroach on the foveal center. Widefield FAF imaging is helpful to elucidate the extent of involved tissue.[11]
Optical coherence tomography shows hyperreflective nodules at the level of the RPE that colocalize with macular pigment clumps on color fundus photography, hyperautofluorescence on FAF, and hyperreflectance on NIR imaging.[7][11][12] Unlike typical drusen or subretinal drusenoid deposits, these lesions appear to reside at the level of the RPE and project a shadow onto the underlying choroid.[11][33] These lesions may not be present on macular OCT in late-stage atrophic disease.[11] Although there may be ill-defined irregularity in the outer retinal bands, there is no clear OCT correlate for yellow macular deposits or the hypoautofluorescent component of FAF lesions.[11][12] OCT angiography may be demonstrate choriocapillaris flow deficits, which may precede other imaging abnormalities in individuals with high PPS exposure.[13][14]
Near infrared reflectance imaging is another important imaging modality with prominent findings that may be present even in the absence of visible lesions in other imaging modalities.[12] Nodular hyperreflective lesions are visible on NIR that correspond to the pigmented lesions on color fundus photography and hyperreflective RPE excrescences on OCT.[7][11][12] Centrifugal progression of parafoveal hyperreflective lesions are noted which develop into hyporeflective areas due to EZ attenuation and RPE atrophy over time on NIR. [34]
Some functional visual tests may also be useful in detecting visual dysfunction not apparent with BCVA testing alone. Dark adaptometry, perimetry, electroretinography, and visual function questionnaires such as the National Eye Institute Visual Function Questionnaire-39 and the Low Luminance Questionnaire have shown profound visual function deficits in PPS maculopathy patients that was not fully captured with BCVA testing.[7][11][32][35]
Laboratory test
There are no indicated laboratory tests for PPS maculopathy at this time. In cases of diagnostic uncertainty, genetic testing to rule out inherited retinal diseases may be helpful, although these can often be distinguished by conventional fundus imaging.[36]
Differential diagnosis
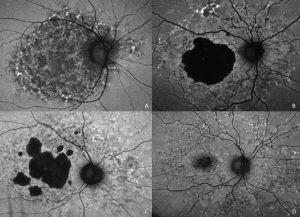
The differential diagnosis for PPS maculopathy includes age-related macular degeneration (AMD), macular dystrophies including pattern dystrophies, maternally inherited diabetes and deafness syndrome (MIDD),[37] and pachychoroid pigment epitheliopathy. In most cases, it is possible, and clinically useful, to be able to differentiate these conditions without relying on the history of prior PPS exposure. Indeed, not all patients with macular lesions who take PPS have PPS maculopathy. Additionally, not all patients will disclose a past history of PPS use.
Age-related macular degeneration can be differentiated most readily by looking for the characteristic pattern of PPS maculopathy on FAF imaging, as described above.[33] Additionally, the hyperreflectant RPE lesions observed in earlier stages of PPS maculopathy are distinct from drusen and subretinal drusenoid deposits of AMD. In PPS maculopathy, these lesions appear to be at the level of the RPE and project a shadow onto the underlying choroid, and colocalize with highly hyperreflectant lesions on NIR imaging.[33] In one study comparing PPS maculopathy to AMD, there were no typical macular drusen in eyes with PPS maculopathy.[33] In patients found to have PPS maculopathy, there was a significantly greater likelihood of having pigment clumps at the level of the RPE as compared to AMD.[33]
Hereditary macular and pattern dystrophies typically have less densely-packed FAF lesions than PPS maculopathy and typically spare involvement of the autofluorescence signal in the peripapillary tissue.[38][39] This contrasts to the peripapillary hypoautofluorescent halo common in PPS maculopathy.[36] Another distinguishing characteristic of pattern dystrophies is that they are associated with mutations in a number of genes that are classically inherited in an autosomal dominant pattern.
Mitochondrial disorders such as MIDD can also cause a pigmentary maculopathy but the lesions characteristic of MIDD typically spare the fovea until late disease (unlike PPS maculopathy). Most cases with MIDD-associated macular changes have systemic manifestations such as deafness and diabetes mellitus.[40]
Pachychoroid diseases may present with similar macular changes as PPS maculopathy but characteristically have additional findings such as dilated choroidal vessels, choroidal hyperpermeability, RPE and neurosensory retinal detachments, and streaks from prior exudation on FAF imaging.[41] Pachychoroid diseases may also present asymmetrically between eyes while PPS maculopathy is typically symmetric. [11][41]
Management
There is currently no known treatment for PPS maculopathy. Primary prevention through avoidance or minimizing the cumulative exposure to PPS appears to be key to preventing the condition. Affected patients should undergo continued monitoring for treatable complications of PPS maculopathy such as CME and MNV, as outlined below.
General treatment
Prescribers of PPS should consider counseling patients regarding the risk of vision loss associated with PPS and have a discussion weighing risks and benefits of taking PPS and potential alternative therapies. If PPS is prescribed, providers should prescribe the minimum dose and duration necessary for disease management.
After a diagnosis of PPS maculopathy, patients are encouraged to work with their prescriber to transition to other IC therapies. Avoidance of smoking and protection from ultraviolet radiation may be beneficial, as in other degenerative maculopathies. After diagnosis, providers should consider monitoring patients annually with multimodal retinal imaging so that treatable vision-threatening sequelae may be addressed should they occur. Pentosan polysulfate maculopathy may progress after drug cessation.[31][42] In eyes with RPE atrophy, there is progressive growth of the atrophic lesions after drug cessation. Additionally, some eyes can develop new onset atrophy after drug cessation.[35][42]
Lastly, a study by Jung et al. has identified a potential novel association between PPS use and colopathy. While the causal relationship and exact pathophysiology remain unclear, the presence of colonic dysplasia in some patients raises concerns about potential deleterious outcomes. These findings underscore the importance of vigilant screening by providers managing patients on PPS.[43]
Medical therapy
There is no known treatment for PPS maculopathy itself. Affected individuals should consult with their ophthalmologist and PPS prescriber to discuss drug cessation and explore alternative treatments for IC.
Some vision-threatening complications of PPS maculopathy, including CME and MNV, can be treated pharmacologically. CME can be treated with topical carbonic anhydrase inhibitors, corticosteroids, or nonsteroidal anti-inflammatory agents; oral acetazolamide; or intravitreal anti-VEGF injections. Macular neovascularization can also be treated with intravitreal anti-VEGF therapy.[16][21] The maculopathy may worsen after discontinuation of Pentosan Polysulfate which suggests that early screening is important. [44]
Medical follow up
Ophthalmologists at the Emory Eye Center monitor patients taking PPS or diagnosed with PPS maculopathy annually with a comprehensive retinal evaluation including color fundus photography, FAF, OCT, and NIR imaging.
Another group recommends an initial exam within six months of initiating PPS and annual exams as the patient approaches 500 g cumulative exposure. Another study highlights the importance of increasing awareness among primary care physicians that may be providing prescription renewals.[30]
Complications
Retinal pigment epithelium atrophy appears to be a manifestation of advanced PPS maculopathy.[11][12] It typically first develops as parafoveal multifocal atrophy that may coalesce over time and involve the foveal center.[11]
Leaking and non-leaking CME has been described in PPS maculopathy and has been successfully treated with both topical and intravitreal therapies described above.[11][15] Macular neovascularization has been reported in PPS maculopathy patients and has been successfully treated with intravitreal anti-VEGF injections.[11][16]
Prognosis
Although more longitudinal data is needed to explore the long term prognosis of this recently discovered condition, PPS maculopathy is not known to be reversible and may progress after drug cessation.[31][42] These findings suggest that early detection is beneficial.[35] Additionally, patients with PPS maculopathy should undergo continued monitoring for treatable complications including CME and CNV.
References
- ↑ Jump up to: 1.0 1.1 Hanno PM, Erickson D, Moldwin R, Faraday MM, American Urological Association. Diagnosis and treatment of interstitial cystitis/bladder pain syndrome: AUA guideline amendment. J Urol. 2015;193(5):1545-1553. doi:10.1016/j.juro.2015.01.086
- ↑ Parsons, C. L., Schmidt, J. D., & Pollen, J. J. (1983). Successful treatment of interstitial cystitis with sodium pentosan polysulfate. J. Urol., 130(1), 51–53. https://doi.org/10.1016/s0022-5347(17)50948-9
- ↑ Clemens, J. Q., Erickson, D. R., Varela, N. P., & Lai, H. H. (2022). Diagnosis and Treatment of Interstitial Cystitis/Bladder Pain Syndrome. Journal of Urology, 208(1), 34–42. https://doi.org/10.1097/JU.0000000000002756
- ↑ Jump up to: 4.0 4.1 4.2 4.3 4.4 4.5 4.6 4.7 Janssen Pharmaceutical Companies. (2020). Elmiron (Pentodan Polysulfate Sodium) Capsules Prescribing Information.
- ↑ Malde, S., Palmisani, S., Al-Kaisy, A., & Sahai, A. (2018). Guideline of guidelines: bladder pain syndrome. BJU Int., 122(5), 729–743. https://doi.org/10.1111/bju.14399
- ↑ Giusto, L. L., Zahner, P. M., & Shoskes, D. A. (2018). An evaluation of the pharmacotherapy for interstitial cystitis. Expert Opin. Pharmacother., 19(10), 1097–1108. https://doi.org/10.1080/14656566.2018.1491968
- ↑ Jump up to: 7.00 7.01 7.02 7.03 7.04 7.05 7.06 7.07 7.08 7.09 7.10 7.11 7.12 7.13 7.14 Pearce, W. A., Chen, R., & Jain, N. (2018). Pigmentary maculopathy associated with chronic exposure to pentosan polysulfate sodium. Ophthalmology, 125(11), 1793–1802. https://doi.org/10.1016/j.ophtha.2018.04.026
- ↑ Jump up to: 8.0 8.1 8.2 Hanif, A. M., Shah, R., Yan, J., Varghese, J. S., Patel, S. A., Cribbs, B. E., O’Keefe, G., Hendrick, A. M., Shantha, J. G., Hubbard 3rd, G. B., Patel, P. S., Rao, P., Yeh, S., & Jain, N. (2019). Strength of association between pentosan polysulfate and a novel maculopathy. Ophthalmology, 126(10), 1464–1466. https://doi.org/10.1016/j.ophtha.2019.04.024
- ↑ Kalbag, N. S., Maganti, N., Lyon, A. T., & Mirza, R. G. (2021). Maculopathy secondary to pentosan polysulfate use: A single-center experience. Clin. Ophthalmol., 15, 513–519. https://doi.org/10.2147/OPTH.S285013
- ↑ Jump up to: 10.0 10.1 10.2 10.3 Vora, R. A., Patel, A. P., & Melles, R. (2020). Prevalence of maculopathy associated with long-term pentosan polysulfate therapy. Ophthalmology, 127(6), 835–836. https://doi.org/10.1016/j.ophtha.2020.01.017
- ↑ Jump up to: 11.00 11.01 11.02 11.03 11.04 11.05 11.06 11.07 11.08 11.09 11.10 11.11 11.12 11.13 11.14 11.15 11.16 11.17 11.18 11.19 11.20 11.21 11.22 11.23 11.24 11.25 11.26 11.27 11.28 11.29 11.30 11.31 11.32 11.33 Hanif, A. M., Armenti, S. T., Taylor, S. C., Shah, R. A., Igelman, A. D., Jayasundera, K. T., Pennesi, M. E., Khurana, R. N., Foote, J. E., O’Keefe, G. A., Yang, P., Hubbard 3rd, G. B., Hwang, T. S., Flaxel, C. J., Stein, J. D., Yan, J., & Jain, N. (2019). Phenotypic spectrum of pentosan polysulfate sodium-associated maculopathy: A multicenter study: A multicenter study. JAMA Ophthalmol., 137(11), 1275–1282. https://doi.org/10.1001/jamaophthalmol.2019.3392
- ↑ Jump up to: 12.00 12.01 12.02 12.03 12.04 12.05 12.06 12.07 12.08 12.09 12.10 12.11 12.12 12.13 12.14 12.15 12.16 12.17 12.18 12.19 12.20 12.21 12.22 12.23 12.24 Wang, D., Velaga, S. B., Grondin, C., Au, A., Nittala, M., Chhablani, J., Vupparaboina, K. K., Gunnemann, F., Jung, J., Kim, J.-H., Ip, M., Sadda, S., & Sarraf, D. (2021). Pentosan polysulfate maculopathy: Prevalence, spectrum of disease, and choroidal imaging analysis based on prospective screening. Am. J. Ophthalmol., 227, 125–138. https://doi.org/10.1016/j.ajo.2021.02.025
- ↑ Jump up to: 13.0 13.1 13.2 Abou-Jaoude, M. M., Davis, A. M., Fraser, C. E., Leys, M., Hinkle, D., Odom, J. V., & Maldonado, R. S. (2021). New insights into pentosan polysulfate maculopathy. Ophthalmic Surg. Lasers Imaging Retina, 52(1), 13–22. https://doi.org/10.3928/23258160-20201223-04
- ↑ Jump up to: 14.0 14.1 14.2 Fogel Levin, M., Santina, A., Corradetti, G., Au, A., Lu, A., Abraham, N., Somisetty, S., Romero Morales, V., Wong, A., Sadda, S., & Sarraf, D. (2022). Pentosan polysulfate sodium-associated maculopathy: Early detection using OCT angiography and choriocapillaris flow deficit analysis. Am. J. Ophthalmol., 244, 38–47. https://doi.org/10.1016/j.ajo.2022.07.015
- ↑ Jump up to: 15.0 15.1 15.2 15.3 15.4 De Larochellière, E., & Bourgault, S. (2022). Pentosan polysulfate sodium-induced pigmentary maculopathy with nonleaking cystoid macular edema successfully treated with anti–vascular endothelial growth factor therapy. Retin. Cases Brief Rep., 16(4), 482–485. https://doi.org/10.1097/icb.0000000000001013
- ↑ Jump up to: 16.0 16.1 16.2 16.3 16.4 16.5 16.6 Mishra, K., Patel, T. P., & Singh, M. S. (2020). Choroidal neovascularization associated with pentosan polysulfate toxicity. Ophthalmol. Retina, 4(1), 111–113. https://doi.org/10.1016/j.oret.2019.08.006
- ↑ Bae, S. S., Sodhi, M., Maberley, D., Kezouh, A., & Etminan, M. (2022). Risk of maculopathy with pentosan polysulfate sodium use. Br. J. Clin. Pharmacol., 88(7), 3428–3433. https://doi.org/10.1111/bcp.15303
- ↑ Hadad, A., Helmy, O., Leeman, S., & Schaal, S. (2020). A novel multimethod image analysis to quantify pentosan polysulfate sodium retinal toxicity. Ophthalmology, 127(3), 429–431. https://doi.org/10.1016/j.ophtha.2019.10.013
- ↑ Jain, N., Li, A. L., Yu, Y., & VanderBeek, B. L. (2020). Association of macular disease with long-term use of pentosan polysulfate sodium: findings from a US cohort. Br. J. Ophthalmol., 104(8), 1093–1097. https://doi.org/10.1136/bjophthalmol-2019-314765
- ↑ Leung, E. H., Sharma, S., Levie-Sprick, A., Lee, G. D., Cho, H., & Mukkamala, K. (2021). Pentosan polysulfate sodium-associated pigmentary retinopathy: Risk factors and fundus findings. Clin. Ophthalmol., 15, 4809–4816. https://doi.org/10.2147/OPTH.S340041
- ↑ Jump up to: 21.0 21.1 21.2 21.3 Jain, N., Liao, A., Garg, S. J., Patel, S. N., Wykoff, C. C., Yu, H. J., London, N. J. S., Khurana, R. N., Zacks, D. N., Berrocal, A. M., Lujan, B. J., Greenlee, T. E., Singh, R. P., Hendrick, A., Jain, N., Liao, A., Meyer, B. I., O’Keefe, G. A., Shah, R., … Singh, M. S. (2022). Expanded Clinical Spectrum of Pentosan Polysulfate Maculopathy: A Macula Society Collaborative Study. Ophthalmology Retina, 6(3), 219–227. https://doi.org/10.1016/j.oret.2021.07.004
- ↑ Lindeke-Myers, A., Hanif, A. M., & Jain, N. (2022). Pentosan polysulfate maculopathy. Surv. Ophthalmol., 67(1), 83–96. https://doi.org/10.1016/j.survophthal.2021.05.005
- ↑ Clark, S. J., Keenan, T. D. L., Fielder, H. L., Collinson, L. J., Holley, R. J., Merry, C. L. R., van Kuppevelt, T. H., Day, A. J., & Bishop, P. N. (2011). Mapping the differential distribution of glycosaminoglycans in the adult human retina, choroid, and sclera. Invest. Ophthalmol. Vis. Sci., 52(9), 6511–6521. https://doi.org/10.1167/iovs.11-7909
- ↑ Ishikawa, M., Sawada, Y., & Yoshitomi, T. (2015). Structure and function of the interphotoreceptor matrix surrounding retinal photoreceptor cells. Exp. Eye Res., 133, 3–18. https://doi.org/10.1016/j.exer.2015.02.017
- ↑ Girardot PE, Zhang X, Zhang N, Donaldson KJ, Chrenek MA, Sellers JT, Feola AJ, Papania J, Nickerson JM, Jain N, Boatright JH. Pentosan Polysulfate Sodium Causes Diminished Function and Subtle Morphological Changes in Retina and RPE of Mice. Invest Ophthalmol Vis Sci. 2024 Feb 1;65(2):28. doi: 10.1167/iovs.65.2.28. Erratum in: Invest Ophthalmol Vis Sci. 2024 Apr 1;65(4):47. doi: 10.1167/iovs.65.4.47. PMID: 38381414; PMCID: PMC10893900.
- ↑ Greenlee, T., Hom, G., Conti, T., Babiuch, A. S., & Singh, R. (2019). Re: Pearce et al.: Pigmentary maculopathy associated with chronic exposure to pentosan polysulfate sodium (Ophthalmology. 2018;125:1793-1802). Ophthalmology, 126(7), e51. https://doi.org/10.1016/j.ophtha.2018.12.037
- ↑ Leschey, K. H., Hines, J., Singer, J. H., Hackett, S. F., & Campochiaro, P. A. (1991). Inhibition of growth factor effects in retinal pigment epithelial cells. Invest. Ophthalmol. Vis. Sci., 32(6), 1770–1778.
- ↑ Girardot, P., Zhang, X., & Gefke, I. (2019). Systemic Pentosan Polysulfate Administration Causes Retinal Function Loss in the C57Bl/6J Mouse. 60(9):2352-2352.
- ↑ Girardot PE, Zhang X, Zhang N, et al. Pentosan Polysulfate Sodium Causes Diminished Function and Subtle Morphological Changes in Retina and RPE of Mice. Invest Ophthalmol Vis Sci. 2024 Feb 1;65(2):28.
- ↑ Jump up to: 30.0 30.1 Astafurov, K. V, Bakri, S. J., & Barkmeier, A. J. (2021). Ocular toxicity associated with pentosan polysulfate sodium. Mayo Clin. Proc., 96(6), 1682–1684. https://doi.org/10.1016/j.mayocp.2021.04.002
- ↑ Jump up to: 31.0 31.1 31.2 31.3 31.4 31.5 Huckfeldt, R. M., & Vavvas, D. G. (2019). Progressive maculopathy after discontinuation of pentosan polysulfate sodium. Ophthalmic Surg. Lasers Imaging Retina, 50(10), 656–659. https://doi.org/10.3928/23258160-20191009-10
- ↑ Jump up to: 32.0 32.1 Lyons, R., Hanif, A., & Jain, N. (2020). Pentosan Polysulfate Maculopathy: Comprehensive Functional Analysis and Structure-Function Correlation. Invest Ophthalmol Vis Sci, 61(7), 1068–1068.
- ↑ Jump up to: 33.0 33.1 33.2 33.3 33.4 Christiansen, J. S., Barnes, A. C., Berry, D. E., & Jain, N. (2022). Pentosan polysulfate maculopathy versus age-related macular degeneration: comparative assessment with multimodal imaging. Canadian Journal of Ophthalmology, 57(1), 16–22. https://doi.org/10.1016/j.jcjo.2021.02.007
- ↑ Santina A, Fogel-Levin M, Abraham N, Sarraf D. NINE-YEAR PROGRESSION OF PENTOSAN MACULOPATHY WITH MULTIMODAL RETINAL IMAGING. Retin Cases Brief Rep. 2023 Nov 1;17(6):664-667. doi: 10.1097/ICB.0000000000001276. PMID: 35344532.
- ↑ Jump up to: 35.0 35.1 35.2 Jung, E. H., Lindeke-Myers, A., & Jain, N. (2023). Two-year outcomes after variable duration of drug cessation in patients with maculopathy associated with pentosan polysulfate use. JAMA Ophthalmol., 141(3), 260–266. https://doi.org/10.1001/jamaophthalmol.2022.6093
- ↑ Jump up to: 36.0 36.1 Barnes, A. C., Hanif, A. M., & Jain, N. (2020). Pentosan polysulfate maculopathy versus inherited macular dystrophies: Comparative assessment with multimodal imaging. Ophthalmol. Retina, 4(12), 1196–1201. https://doi.org/10.1016/j.oret.2020.05.008
- ↑ Tripathy, K., Sarma, B., & Mazumdar, S. (2020). Outer retinal tubulation and inner retinal pseudocysts in a patient with maternally inherited diabetes and deafness evaluated with optical coherence tomography angiogram. Indian J. Ophthalmol., 68(1), 250–253. https://doi.org/10.4103/ijo.IJO_577_19
- ↑ Cideciyan, A. V, Swider, M., Aleman, T. S., Sumaroka, A., Schwartz, S. B., Roman, M. I., Milam, A. H., Bennett, J., Stone, E. M., & Jacobson, S. G. (2005). ABCA4-associated retinal degenerations spare structure and function of the human parapapillary retina. Invest. Ophthalmol. Vis. Sci., 46(12), 4739–4746. https://doi.org/10.1167/iovs.05-0805
- ↑ Duncker, T., Tsang, S. H., Woods, R. L., Lee, W., Zernant, J., Allikmets, R., Delori, F. C., & Sparrow, J. R. (2015). Quantitative fundus autofluorescence and optical coherence tomography in PRPH2/RDS- and ABCA4-associated disease exhibiting phenotypic overlap. Invest. Ophthalmol. Vis. Sci., 56(5), 3159–3170. https://doi.org/10.1167/iovs.14-16343
- ↑ Hanif, A. M., Yan, J., & Jain, N. (2019). Pattern dystrophy: An imprecise diagnosis in the age of precision medicine. Int. Ophthalmol. Clin., 59(1), 173–194. https://doi.org/10.1097/IIO.0000000000000262
- ↑ Jump up to: 41.0 41.1 Warrow, D. J., Hoang, Q. V, & Freund, K. B. (2013). Pachychoroid pigment epitheliopathy. Retina, 33(8), 1659–1672. https://doi.org/10.1097/IAE.0b013e3182953df4
- ↑ Jump up to: 42.0 42.1 42.2 Shah, R., Simonett, J. M., Lyons, R. J., Rao, R. C., Pennesi, M. E., & Jain, N. (2020). Disease course in patients with pentosan polysulfate sodium-associated maculopathy after drug cessation. JAMA Ophthalmol., 138(8), 894–900. https://doi.org/10.1001/jamaophthalmol.2020.2349
- ↑ Jung EH, Zheng W, Weiss RJ, et al. Colopathy Associated with Pentosan Polysulfate Use. Published online April 4, 2023:2023.04.03.23288071. doi:10.1101/2023.04.03.23288071
- ↑ Mukhopadhyay C, Boyce TM, Gehrs KM, Folk JC, Mullins RF, Luo Y, Kreder K, Sohn EH. Age-Related Macular Degeneration Masquerade: A Review of Pentosan Polysulfate Maculopathy and Implications for Clinical Practice. Asia Pac J Ophthalmol (Phila). 2022 Mar-Apr 01;11(2):100-110.

