Pelizaeus-Merzbacher Disease
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
Pelizaeus-Merzbacher disease (PMD) is a rare, X-linked recessive hypomyelinating leukodystrophy characterized by nystagmus, motor developmental delays, and spasticity.[1] PMD arises from a variety of mutations in the proteolipid protein 1 (PLP1) gene that produce an abnormal membrane protein, causing aberrant myelination and oligodendrocyte function.
Disease Entity
Disease
As an X-linked recessive condition, males are mainly affected by PMD.[1] Females are mainly carriers and heterozygous females experience no neurological symptoms. PMD is an uncommon condition. The incidence of PMD is as follows:[1]
- 1 in 90,000 to 1 in 750,000 globally
- 1.9 in 100,000 within the United States
Etiology
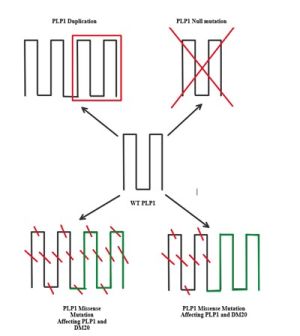
PMD is due to an X-linked recessive mutation in the PLP1 gene (e.g., duplication, missense, and deletion).[1] These mutations eventually lead to a reduction or, in some cases, complete elimination of myelin production in affected individuals. This result is likely due to the critical role of PLP1 in producing the myelin sheath by encoding a major protein component known as proteolipid protein (PLP).[3]
Pathophysiology
Mutations in PLP1 have varying mechanisms of impact depending on the type of mutation that occurred.[1] Point mutations result in misfolding of PLP and, later, impaired passage of the protein through the Golgi apparatus—which is responsible for protein modification. Subsequently, it amasses in the endoplasmic reticulum (ER). Hindrance of the ER leads to decreased protein production and eventual toxicity in oligodendrocytes, resulting in reduced myelin production.
Null mutations, those that result in premature stop codons and shortened protein products, do not accumulate in the ER, resulting in far fewer oligodendrocyte casualties.[1] However, decreased myelin production has been observed in individuals with a null mutation. Therefore, the end product of this mutation is a mild phenotype. Individuals with deletion mutations share a similar phenotype with a similar underlying mechanism. However, missense mutations result in the most severe presentation because they lead to eventual oligodendrocyte apoptosis and axonal injury. Mutations in DM20, a protein isoform in close proximity to PLP1, also play a key role in determining disease severity.
Risk Factors
Due to the X-linked recessive nature of inheritance, key risk factors for PMD include:[1]
- Family history of the condition
- Presence of a mutated copy of the PLP1 gene in the patient’s mother
- Presence of a mild form of the mutated PLP1 gene in the patient’s father
- Male sex
Diagnosis
History
There are three documented forms of PMD.[1] Type I is the most severe with connatal involvement while type III, known as the classic type, is the least severe. Type II, also known as the transitional type, falls in between I and III in terms of severity. Type III PMD initially presents with symptoms at around 1 year old. These patients become spastic but are able to maintain limited ambulation and remain intact cognitively. In contrast, Type I patients present with symptoms upon birth. These patients are unable to ambulate, unable to speak, and are significantly impaired cognitively later in life, manifesting as delayed acquisition of developmental milestones. Patients also endorse a history of seizures. Type II PMD bears features of both type I and type III. Regardless of the clinical phenotype, caregivers of children with PMD reported that most of the disease symptoms present before 2 years of age.[4]
Physical examination
According to a case series conducted by Velasco Parra et al. in Colombia including patients ranging from 6 months to 16 years old, features common to all documented forms of PMD include:[5]
- Horizontal nystagmus (57%)
- Rotary nystagmus (43%)
- Cerebellar signs (71.4%)
- Spasticity (85.7%)
Observed findings outside of the case series include:
Type I, being more severe, demonstrates further findings including:[1]
- Cognitive delay
- Inability to ambulate
- Laryngeal stridor
- Pharyngeal weakness
- Respiratory failure
Ocular Findings
Ocular Findings in PMD can include:
- Horizontal and Rotary Nystagmus[1]
- Poor spatial vision[6]
- Poor visual field[6]
- Optic nerve enlargement (observed in one case study)[7]
- Diminished vestibulo-ocular reflex[8]
Laboratory Testing and Imaging
Imaging studies are critical in diagnosing PMD. Key imaging modalities for diagnosis include:
- CT demonstrates white matter attenuation and progressive atrophy[1]
- MRI demonstrates hypomyelination and can indicate connatal form[1]
- Diffuse hyperintensities on T2 MRI over many areas, including:
- Posterior limb of internal capsule
- Optical radiations
- Corona radiata
- Cerebellar hypoplasia
- In one case observed by Pavlidou et al., optic nerve enlargement was also observed on MRI.[7]
- Diffuse hyperintensities on T2 MRI over many areas, including:
More recently, Harting et al proposed an MRI myelination scoring system to allow for the stratification of PMD patients and monitor changes in myelination (Table 1). The myelination scoring system comprises scoring 8 T2-and 6 T1-weighted images from specific anatomic sites on a scale of 0-2 according to the degree of signal intensity relative to the cortex.[9] Implementing a myelination score may provide a more objective standardized assessment during patient follow-up.[9]
Table 1. Brain myelination score for PMD.[9]
| PMD Myelination Score | Item (Anatomic Site) | Reference | T2w | T1w |
|---|---|---|---|---|
| Pyramidal Tract | Central region | Cortexα | 0-2 | 0-2 |
| Central semiovale | Cortexα | 0-2 | 0-2 | |
| PLIC | Cortex | 0-2 | 0-2 | |
| Visual Tract | Optic radiation | Cortex | 0-2 | 0-2 |
| Primary visual cortex | Cortex | 0-2 | 0-2 | |
| MCP | Cortex | 0-2 | 0-2 | |
| Other | Frontal (not central) | Cortex | 0-2 | - |
| Medial lemniscus | Surrounding | 0-1 | - | |
| Sum | 0-15 | 0-12 | ||
| Myelination Score | 0-27 |
Lab tests to confirm the diagnosis include genetic tests such as:[1]
- Fluorescence in-situ hybridization (FISH)
- Multiplex ligation-dependent probe amplification (MLPA)
- Chromosomal microarray
- Droplet-digital polymerase chain reaction (dd-PCR)
Differential diagnosis
The differential diagnosis for PMD includes:
- Metachromatic leukodystrophy[1]
- Adrenoleukodystrophy[1]
- Pelizaeus-Merzbacher-like disease (PMLD)[1]
- Spastic Paraplegia Type II[1]
- PLP1-null disease[2]
Management
At this time, there is no treatment that can completely cure PMD. Most treatment modalities revolve around palliative care and symptom relief.[1] According to a survey of caregivers of individuals with PMD, increasing mobility and communication were the most important treatment goals.[4] Current therapies include the use of skeletal muscle relaxants like baclofen, diazepam, or tizanidine to address spasticity, a gastrotomy to assist with pharyngeal weakness with subsequent enteral feeding, and physical therapy to address scoliosis in some patients.
Dietary interventions may also be effective in reducing the severity of PMD. Specifically, a diet rich in cholesterol was capable of increasing the life span of oligodendrocytes according to a study conducted by Saher et al.[10] Furthermore, Stumpf et al tested a ketogenic diet, one that is high in fat and low in carbohydrates, in mouse models and observed the regeneration of oligodendrocytes.[11] Additional mouse model studies have shown a potential benefit with curcumin, although a study of administration of an open-label bioavailable form of curcumin in nine patients with PMD failed to demonstrate any significant therapeutic effects after 12 months.[12]
Although there are no definitive treatments available to cure PMD, there are some treatments in development that can target its molecular pathology. One such treatment uses the CRISPR (clustered regularly interspaced short palindromic repeats)-Cas9 system in mouse models to suppress the expression of PLP1, resulting in a phenotype similar to the mild form.[13] Other treatments focus on reducing the expression of PLP1 through pharmacological interventions. Lonaprisan is an example of a progesterone receptor antagonist that decreases PLP1 expression.[1] ER stress modulators and iron chelation as well as several other novel therapies will be trialed in PMD patients soon including RNA-suppressive therapy and glial progenitor cell transplantation.[14]
The prognosis of PMD patients depends on the form that they present with. If left untreated, patients with Type I PMD would not survive past childhood due to respiratory failure. [1] However, with intervention, these patients can survive up to the third decade of life. Patients diagnosed with Type III PMD can live as long as the seventh decade of life.
Increased awareness of the cardinal features of PMD will aid clinicians in recognizing the different PMD types. Nystagmus and poor vision are the predominant presentations of PMD. Although there is no cure currently for PMD, future research is ongoing to reduce or mitigate the PLP1 mutation effects.
References
- ↑ 1.00 1.01 1.02 1.03 1.04 1.05 1.06 1.07 1.08 1.09 1.10 1.11 1.12 1.13 1.14 1.15 1.16 1.17 1.18 1.19 1.20 Singh, R., & Samanta, D. (2021). Pelizaeus-Merzbacher Disease. In StatPearls [Internet]. StatPearls Publishing.
- ↑ 2.0 2.1 Osório, M. J., & Goldman, S. A. (2018). Neurogenetics of Pelizaeus-Merzbacher disease. Handbook of clinical neurology, 148, 701–722. https://doi.org/10.1016/B978-0-444-64076-5.00045-4
- ↑ 3.0 3.1 3.2 3.3 3.4 Hobson, G. M., & Garbern, J. Y. (2012). Pelizaeus-Merzbacher disease, Pelizaeus-Merzbacher-like disease 1, and related hypomyelinating disorders. Seminars in neurology, 32(1), 62–67. https://doi.org/10.1055/s-0032-1306388
- ↑ 4.0 4.1 Moore KM, Wolf NI, Hobson G, et al. Pelizaeus-Merzbacher Disease: A Caregiver Assessment of Disease Impact. J Child Neurol. 2023;38(1-2):78-84. doi:10.1177/08830738231152658
- ↑ Velasco Parra HM, Maradei Anaya SJ, Acosta Guio JC, Arteaga Diaz CE, Prieto Rivera JC. Clinical and mutational spectrum of Colombian patients with Pelizaeus Merzbacher Disease. Colomb Med (Cali). 2018 Jun 30;49(2):182-187.
- ↑ 6.0 6.1 Apkarian, P., Koetsveld-Baart, J. C., & Barth, P. G. (1993). Visual evoked potential characteristics and early diagnosis of Pelizaeus-Merzbacher disease. Archives of neurology, 50(9), 981-985.
- ↑ 7.0 7.1 Pavlidou, E., Ramachandran, V., Govender, V., Wilson, C., Das, R., Vlachou, V., ... & Kinali, M. (2017). A novel PLP1 mutation associated with optic nerve enlargement in two siblings with Pelizaeus–Merzbacher disease: A new MRI finding. Brain and Development, 39(3), 271-274.
- ↑ Huygen, P. L., Verhagen, W. I., & Renier, W. O. (1992). Oculomotor and vestibular anomalies in Pelizaeus-Merzbacher disease: a study on a kindred with 2 affected and 3 normal males, 3 obligate and 8 possible carriers. Journal of the neurological sciences, 113(1), 17–25. https://doi.org/10.1016/0022-510x(92)90259-n
- ↑ 9.0 9.1 9.2 9.3 9.4 9.5 Harting I, Garbade SF, Rosendaal SD, et al. Identification of PMD subgroups using a myelination score for PMD. Eur J Paediatr Neurol. 2022;41:71-79. doi:10.1016/j.ejpn.2022.10.003
- ↑ Saher, G., Rudolphi, F., Corthals, K., Ruhwedel, T., Schmidt, K. F., Löwel, S., ... & Nave, K. A. (2012). Therapy of Pelizaeus-Merzbacher disease in mice by feeding a cholesterol-enriched diet. Nature medicine, 18(7), 1130-1135.
- ↑ Stumpf SK, Berghoff SA, Trevisiol A, Spieth L, Düking T, Schneider LV, Schlaphoff L, Dreha-Kulaczewski S, Bley A, Burfeind D, Kusch K, Mitkovski M, Ruhwedel T, Guder P, Röhse H, Denecke J, Gärtner J, Möbius W, Nave KA, Saher G. Correction to: Ketogenic diet ameliorates axonal defects and promotes myelination in Pelizaeus-Merzbacher disease. Acta Neuropathol. 2019 Oct;138(4):673-674.
- ↑ Yamamoto A, Shimizu-Motohashi Y, Ishiyama A, et al. An Open-Label Administration of Bioavailable-Form Curcumin in Patients With Pelizaeus-Merzbacher Disease. Pediatr Neurol. 2024;151:80-83. doi:10.1016/j.pediatrneurol.2023.11.014
- ↑ Elitt, M. S., Barbar, L., Shick, H. E., Powers, B. E., Maeno-Hikichi, Y., Madhavan, M., Allan, K. C., Nawash, B. S., Gevorgyan, A. S., Hung, S., Nevin, Z. S., Olsen, H. E., Hitomi, M., Schlatzer, D. M., Zhao, H. T., Swayze, A., LePage, D. F., Jiang, W., Conlon, R. A., Rigo, F., … Tesar, P. J. (2020). Suppression of proteolipid protein rescues Pelizaeus-Merzbacher disease. Nature, 585(7825), 397–403. https://doi.org/10.1038/s41586-020-2494-3
- ↑ Elitt MS, Tesar PJ. Pelizaeus-Merzbacher disease: on the cusp of myelin medicine. Trends Mol Med. 2024;30(5):459-470. doi:10.1016/j.molmed.2024.03.005

