Pediatric Myelin Oligodendrocyte Glycoprotein Antibody-Associated Disease (MOGAD)
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
Myelin Oligodendrocyte Glycoprotein Antibody-Associated Disease (MOGAD) is a demyelinating disease of the central nervous system associated with seropositivity of the MOG antibody. Pediatric MOG has a wide clinical phenotype and MOG antibody testing should be considered in all children with CNS demyelinating disease.[1] The diagnosis of MOGAD is important for counseling patients and their families on relapse risk and available treatments. This article highlights the unique features of MOGAD in the pediatric population rather providing a general summary of MOGAD which has been described elsewhere. This article focuses on five areas which summarize the unique features of MOGAD in children and include:
Pediatric MOGAD is more heterogenous than the adult form of the disease, with the differing features of pediatric disease accentuated in younger patients.
Disease Entity
Epidemiology
The incidence of MOGAD is higher in children than in adults, with a recent study in the Dutch population demonstrating an incidence of 0.31/100,000 of MOG positive acute demyelinating syndromes (ADS) compared with 0.13/100,000 in adults.[2] It is estimated that 50% of all MOGAD cases occur in children.[3] [4] As with adults there does not appear to be a sex-bias with MOGAD with the female-to-male ratio being approximately 1:1.[5] [6]
Diagnosis
Clinical Presentation
The most common presentation of MOGAD in children is acute disseminated encephalomyelitis (ADEM), which makes up around 40%–50% of all presenting cases of pediatric MOGAD.[7] [8] [9] In adults optic neuritis (ON) is the commonest clinical presentation and ADEM makes up only a small proportion of incident cases (~10%–15%).[5]This high rate of ADEM in pediatric cases is predominantly seen in children <11 years of age. The clinical phenotype of first attack in children aged >11 replicates that of adults with ADEM only accounting for 13% of cases.[10] Interestingly this difference in presenting features also applies to reoccurrences. Younger patients who had ADEM as a first ADS are more likely to get reoccurrences presenting as ON as they get older.[11] Optic neuritis presents as unilateral or bilateral visual loss, field loss, color vision impairment and/or painful eye movements.[1] However, the MOGAD-ON disease course in children is milder compared with the course in adults, and children are more likely to experience complete recovery.[12]
The International Pediatric Multiple Sclerosis Study Group defines ADEM with the following criteria and all must be present to fulfil the diagnostic criteria:[13]
- A first polyfocal, clinical CNS event with presumed inflammatory demyelinating cause
- Encephalopathy that cannot be explained by fever
- No new clinical and MRI findings emerge three months or more after the onset
- Brain MRI is abnormal during the acute (three-month) phase.
- Typically on brain MRI:
- Diffuse, poorly demarcated, large (>1–2 cm) lesions involving predominantly the cerebral white matter
- T1 hypointense lesions in the white matter are rare
- Deep grey matter lesions (e.g. thalamus or basal ganglia) can be present
Encephalopathy is therefore necessary for the fulfilment of diagnostic criteria although recognizing encephalopathy in children can be challenging with subtle and fluctuating signs which include behavioral change and alteration in consciousness.[13]
Of the MOGAD pediatric patients (who tend to be >11 years old), who present with ON (MOGAD-ON) the majority have bilateral ON, if not clinically then radiologically.[13] Optic disc edema (swollen optic discs) is common in pediatric MOGAD-ON patients and a higher percentage of AQP4-ON and double seronegative ON pediatric patients have swollen optic discs compared to adults, making swollen optic discs a less useful clinical sign for distinguishing the conditions in children.[1]
Approximately 4% of pediatric patients with MOGAD present with neuromyelitis optica (NMO).[1][14] NMO is characterized as recurrent uni- or bilateral ON and/or longitudinally extensive transverse myelitis or brainstem syndromes such as area postrema syndrome.[14] A European prospective cohort study found that 58% of pediatric NMOSD cases were MOG antibody positive and 17% were AQP4 antibody positive. This contrasts with adults where up to 80% of adult NMOSD patients are seropositive for AQP4 antibodies.[15] As with adults there are several other rare forms of ADS associated with MOGAD including, cortical encephalitis, leukodystrophy-like phenotype and overlap syndromes.[1][16]
There are several other differential diagnoses to consider when considering a diagnosis of pediatric ADEM. This includes multiple sclerosis and AQP4-NMOSD as well as viral encephalitis, CNS vasculitis, mitochondrial disorders, malignancies and hemophagocytic lymphohistiocytosis.[17]
Investigation
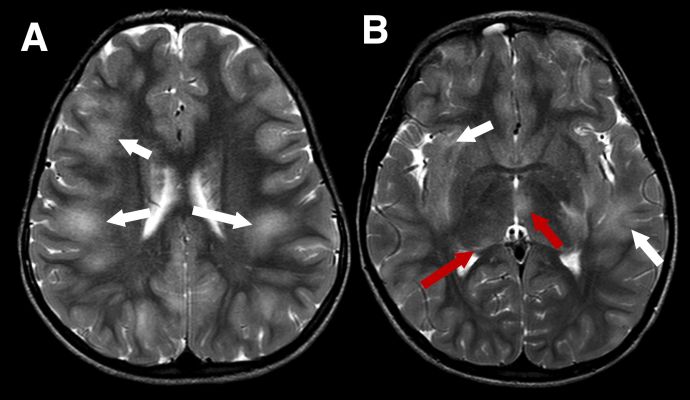
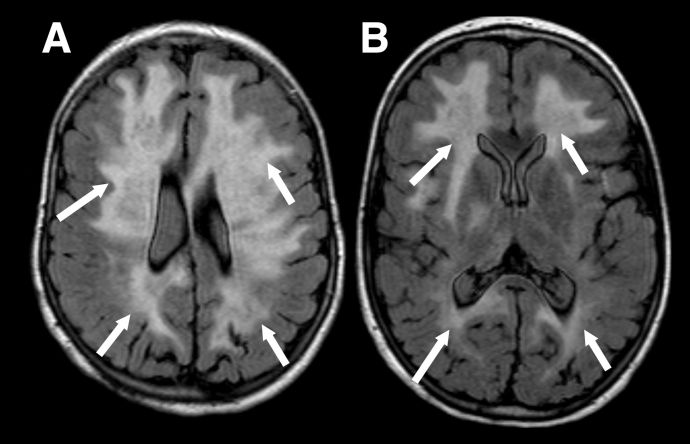
MRI findings depend on presenting phenotype e.g. ADEM vs ON, although there is some crossover. Due to the aforementioned predilection for MOGAD to present as ADEM in younger children the MRI features are inherently different to those seen in adults. MRI features of ADEM are typically bilateral deep white and grey matter lesions which are T2 hyperintense large (>2cm) in size and poorly demarcated (Figure 1). However, MRI findings in pediatric ADEM can demonstrate a “leukodystrophy-like” pattern which includes predominantly confluent and bilateral white matter changes and is associated with a poorer outcome (Figure 2).[18]
Figure 1 Typical imaging appearances in a child with acute disseminated encephalomyelitis (ADEM) MOGAD phenotype in the acute phase. Axial T2-weighted images show large, extensive, ill defined areas of high signal throughout the subcortical white matter and left temporal stem (A and B, white arrows). The thalami are also involved more inferiorly (B, red arrows)
Figure 2 "Leukodystrophy-like" pattern of demyelination. Axial FLAIR images in a child with the leukodystrophy-like MOGAD phenotype showing extensive, bilateral, relatively symmetrical, areas of markedly increased signal throughout the supratentorial white matter (A and B, white arrows).
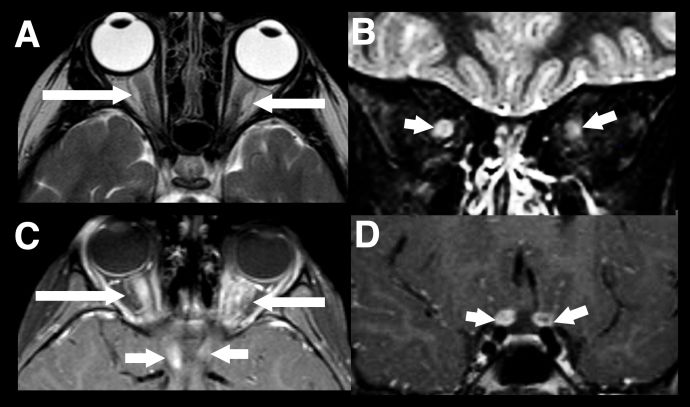
The second most common presentation of pediatric MOGAD is ON however in children radiographic features tend to be bilateral, longitudinally extensive (>1/2 the prechiasmatic optic nerve) an involve the anterior optic nerve which explains the higher incidence of disc swelling (Figure 3).[19] [20]
Figure 3 Longitudinally extensive optic neuritis. Typical imaging findings in a child with acute bilateral MOGAD optic neuritis. There is extensive marked swelling of the intraorbital optic nerve-sheath complexes bilaterally on T2-weighted images, particularly on the right (axial A, and coronal B, white arrows). Marked associated enhancement extending to the prechiasmatic segments of both nerves, are seen on post gadolinium T1-weighted images (axial C and coronal D, white arrows).
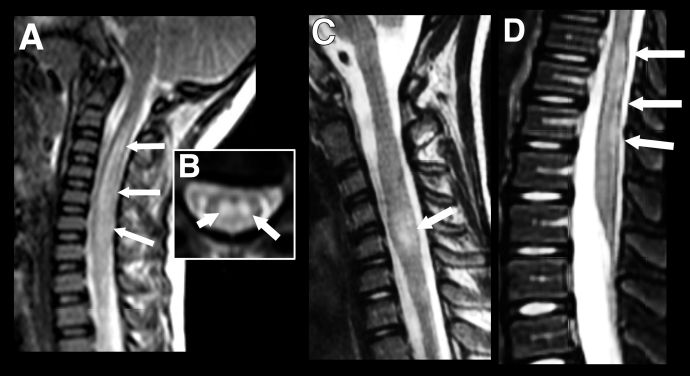
Pediatric patients can present with longitudinally extensive transverse myelitis (LETM) which can occur as part of a wider spectrum of disease or rarely as an isolated presentation. It is defined as a T2-hyperintensity which occurs over three or more vertebral segments (Figure 4). In children it is most often associated with an ADEM phenotype or NMOSD and normally involves the cervical region of the spine.[21] While short (<3 vertebral segments) transverse myelitis has been reported within the context of pediatric MOGAD it is a rarer disease phenotype than that seen in adults (Figure 4). [22] In general, gadolinium enhancement of lesions in pediatric MOGAD patients is less prominent than in adults and, where present, is often diffuse and not confined to discrete, patchy lesions as seen in adults.[23]
Figure 4 Longitudinally extensive transverse myelitis (LETM) vs Short transverse myelitis. Imaging findings in transverse myelitis in pediatric MOGAD in the acute phase. A: Sagittal T2-weighted image showing longitudinally extensive transverse myelitis, with increased signal and marked swelling of the cervical cord. Corresponding mid-cervical axial image (B) shows typical central cord involvement. Comparison case from another child shows a short segment cervical transverse myelitis, but this also involves the central cord (C, sagittal T2-weighted image) as well as a more typical longitudinally extensive transverse myelitis in the lumbar cord (D).
Management
General Treatment
The treatment of pediatric MOGAD in the acute setting is similar to that of adults although some specific considerations apply in children. It is worth noting that many of the treatment trials in MOGAD and demyelinating disease have been conducted in adults and then results are extrapolated to children with no currently published RCT involving exclusively pediatric patients.
A recent systemic review suggested that IVMP is the most commonly used acute treatment (88%) of cases and IVIG the next most common (66%).[24] In the acute setting IV methylprednisolone (IVMP) is commonly used at a dose of 20-30mg/kg daily (max 1g/day) for 3 to 5 days. Most children are steroid responsive with a rapid improvement in visual acuity but a subset of patients do not respond to IVMP. These patients should be rapidly escalated to intravenous immunoglobulins (IVIG) or plasma exchange (PLEX). One retrospective study showed that a short delay to PLEX was the strongest predictor of complete recovery from NMOSD.[25] Other studies have similarly demonstrated that delay to PLEX is predictive of a worse clinical outcome. [24][26] In a retrospective review of 75 children with MOGAD, early initiation of immunotherapy (less than 7 days from symptom onset) was associated with a 6.7-fold reduced risk of relapse.[27] The same study found that a longer steroid taper of 5 weeks or longer also associated with a significantly reduced risk of relapse. There have been no randomized clinical trials of IVIG in acute MOGAD, unreported data suggests it may be beneficial.[28]
As with adults, azathioprine is one of the first line steroid-sparing immunosuppressive agents. In those patients who are intolerant or have a contraindication e.g. have reduced thiopurine methyl transferase (TPMT) activity. An alternative first line agent is myocophenolate mofetil (MMF). It is worth noting that pediatric patients are particularly at risk of relapse during the first 3-6 months of initiation of steroid-sparing immunosuppressive agents and therefore the EU pediatric MOG consortium suggests cotreatment with an oral steroid taper during establishment of long-term immunosuppression.[29]
The long-term risks of both steroid and steroid-sparing agents in pediatric patients is largely the same as those in adults. There are additional considerations on growth with corticosteroids use in children and there is potentially an increased life-time malignancy risk when agents such as azathioprine and MMF are initiated at a young age, although there is no cohort data to support this currently.[29]
The treatment of pediatric patients especially in a condition such as MOGAD which often involves multiple and sometimes lengthy hospital stays has an important psychosocial impact on patients and can affect education. Therefore, pediatric patients require involvement of a pediatric focused multi-disciplinary team including psychological and educational support to maximize quality of life outcomes. Physical and visual disability can uniquely affect pediatric patients at a critical age in development and therefore all attempts must be made to support patients and their families.
Prognosis
Pediatric patients have good recovery, with studies suggesting less long-term disability compared with adults.[30] In pediatric patients, between 75% and 96% were reported to have a complete recovery. Of those patients who present with MOG-ON, there is complete recovery of visual acuity in 56%–73% of patients, which is better than that of adult patients.[20] Pediatric MOG-ON have also been shown to have better recovery compared with adults even when they share similar OCT findings with respect to neuroaxonal loss.[31] Furthermore, within the pediatric patient cohort age, a later age of onset is linearly correlated with a worse visual outcome.[31] Particularly in young patients with MOGAD, there is a predominance of monophasic disease and a good response to initial treatment.[32] Long-term risk of relapse is around 35% but relapse rates in children are lower than that in adults (Hazard ratio 1.42).[3][33]. Early relapses with 12 months of initial onset increase the risk of long-term relapsing disease.[34]
As with adults, a recurrence or multiphasic disease course is associated with a poorer outcome and full recovery is around half that of patients with a monophasic disease course (31-50%).[9] 50% of pediatric patients with multiphasic disseminated encephalomyelitis (MDEM) were found to have cognitive problems in a prospective 2-year study reporting the outcomes of children with relapsing MOGAD.[11] In the same study, no patients with recurrent ON alone (i.e., no episodes of ADEM or NMOSD) developed cognitive problems. However, the risk of visual loss is high in patients with recurrent MOGAD-ON. A diagnosis of MOGAD at the first episode of ON is important to guide management in patients with relapsing disease.
Conclusion
Pediatric MOGAD demonstrates several unique features compared with adult-forms of the diseases. Treatment guidelines have largely been extrapolated from evidence obtained from adult studies, and treatment RCTs are required to clarify whether current pediatric MOGAD treatment practices are optimal.
References
- ↑ Jump up to: 1.0 1.1 1.2 1.3 1.4 Bruijstens AL, Lechner C, Flet-Berliac L, Deiva K, Neuteboom RF, Hemingway C, et al. E.U. paediatric MOG consortium consensus: Part 1 - Classification of clinical phenotypes of paediatric myelin oligodendrocyte glycoprotein antibody-associated disorders. Eur J Paediatr Neurol [Internet]. 2020 Nov 1 [cited 2023 Oct 31];29:2–13. Available from: https://pubmed.ncbi.nlm.nih.gov/33162302/
- ↑ de Mol CL, Wong YYM, van Pelt ED, Wokke BHA, Siepman TAM, Neuteboom RF, et al. The clinical spectrum and incidence of anti-MOG-associated acquired demyelinating syndromes in children and adults. Multiple Sclerosis Journal [Internet]. 2020 Jun 1 [cited 2023 Oct 31];26(7):806–14. Available from: https://journals.sagepub.com/doi/full/10.1177/1352458519845112
- ↑ Jump up to: 3.0 3.1 Cobo-Calvo A, Ruiz A, Rollot F, Arrambide G, Deschamps R, Maillart E, et al. Clinical Features and Risk of Relapse in Children and Adults with Myelin Oligodendrocyte Glycoprotein Antibody-Associated Disease. Ann Neurol [Internet]. 2021 Jan 1 [cited 2023 Oct 31];89(1):30–41. Available from: https://pubmed.ncbi.nlm.nih.gov/32959427/
- ↑ Ramanathan S, Prelog K, Barnes EH, Tantsis EM, Reddel SW, Henderson APD, et al. Radiological differentiation of optic neuritis with myelin oligodendrocyte glycoprotein antibodies, aquaporin-4 antibodies, and multiple sclerosis. Multiple Sclerosis. 2016 Apr 1;22(4):470–82.
- ↑ Jump up to: 5.0 5.1 Sechi E, Cacciaguerra L, Chen JJ, Mariotto S, Fadda G, Dinoto A, et al. Myelin Oligodendrocyte Glycoprotein Antibody-Associated Disease (MOGAD): A Review of Clinical and MRI Features, Diagnosis, and Management. Front Neurol [Internet]. 2022 Jun 17 [cited 2023 Oct 31];13:885218. Available from: /pmc/articles/PMC9247462/
- ↑ Waters P, Fadda G, Woodhall M, O’Mahony J, Brown RA, Castro DA, et al. Serial Anti–Myelin Oligodendrocyte Glycoprotein Antibody Analyses and Outcomes in Children With Demyelinating Syndromes. JAMA Neurol [Internet]. 2020 Jan 1 [cited 2023 Nov 11];77(1):82–93. Available from: /pmc/articles/PMC6763982/
- ↑ Sechi E, Cacciaguerra L, Chen JJ, Mariotto S, Fadda G, Dinoto A, et al. Myelin Oligodendrocyte Glycoprotein Antibody-Associated Disease (MOGAD): A Review of Clinical and MRI Features, Diagnosis, and Management. Front Neurol [Internet]. 2022 Jun 17 [cited 2023 Oct 31];13:885218. Available from: /pmc/articles/PMC9247462/
- ↑ Ketelslegers IA, Van Pelt ED, Bryde S, Neuteboom RF, Catsman-Berrevoets CE, Hamann D, et al. Anti-MOG antibodies plead against MS diagnosis in an Acquired Demyelinating Syndromes cohort. Mult Scler [Internet]. 2015 Oct 1 [cited 2023 Oct 31];21(12):1513–20. Available from: https://pubmed.ncbi.nlm.nih.gov/25662345/
- ↑ Jump up to: 9.0 9.1 Zhou J, Lu X, Zhang Y, Ji T, Jin Y, Xu M, et al. Follow-up study on Chinese children with relapsing MOG-IgG-associated central nervous system demyelination. Mult Scler Relat Disord. 2019 Feb 1;28:4–10.
- ↑ Santoro JD, Beukelman T, Hemingway C, Hokkanen SRK, Tennigkeit F, Chitnis T. Attack phenotypes and disease course in pediatric MOGAD. Ann Clin Transl Neurol [Internet]. 2023 May 1 [cited 2023 Oct 31];10(5):672. Available from: /pmc/articles/PMC10187731/
- ↑ Jump up to: 11.0 11.1 Hacohen Y, Absoud M, Deiva K, Hemingway C, Nytrova P, Woodhall M, et al. Myelin oligodendrocyte glycoprotein antibodies are associated with a non-MS course in children. Neurology(R) neuroimmunology & neuroinflammation [Internet]. 2015 Apr 1 [cited 2023 Oct 31];2(2):e81. Available from: https://pubmed.ncbi.nlm.nih.gov/25798445/
- ↑ Ketelslegers IA, Visser I, Neuteboom RF, Boon M, Catsman-Berrevoets CE, Hintzen RQ. Disease course and outcome of acute disseminated encephalomyelitis is more severe in adults than in children. Mult Scler [Internet]. 2011 [cited 2023 Nov 23];17(4):441–8. Available from: https://pubmed.ncbi.nlm.nih.gov/21148017/
- ↑ Jump up to: 13.0 13.1 13.2 Krupp LB, Tardieu M, Amato MP, Banwell B, Chitnis T, Dale RC, et al. International Pediatric Multiple Sclerosis Study Group criteria for pediatric multiple sclerosis and immune-mediated central nervous system demyelinating disorders: revisions to the 2007 definitions. http://dx.doi.org/101177/1352458513484547 [Internet]. 2013 Apr 9 [cited 2023 Oct 31];19(10):1261–7. Available from: https://journals.sagepub.com/doi/10.1177/1352458513484547
- ↑ Jump up to: 14.0 14.1 Wingerchuk DM, Banwell B, Bennett JL, Cabre P, Carroll W, Chitnis T, et al. International consensus diagnostic criteria for neuromyelitis optica spectrum disorders. Neurology [Internet]. 2015 Jul 1 [cited 2023 Nov 11];85(2):177–89. Available from: https://pubmed.ncbi.nlm.nih.gov/26092914/
- ↑ Lechner C, Baumann M, Hennes EM, Schanda K, Marquard K, Karenfort M, et al. Antibodies to MOG and AQP4 in children with neuromyelitis optica and limited forms of the disease. J Neurol Neurosurg Psychiatry [Internet]. 2016 Jan 8 [cited 2023 Nov 11];87(8):897–905. Available from: https://pubmed.ncbi.nlm.nih.gov/26645082/
- ↑ Kannan V, Sandweiss AJ, Erickson TA, Yarimi JM, Ankar A, Hardwick VA, et al. Fulminant Anti-Myelin Oligodendrocyte Glycoprotein-Associated Cerebral Cortical Encephalitis: Case Series of a Severe Pediatric Myelin Oligodendrocyte Glycoprotein Antibody-Associated Disease Phenotype. Pediatr Neurol [Internet]. 2023 Oct 1 [cited 2023 Nov 23];147:36–43. Available from: https://pubmed.ncbi.nlm.nih.gov/37544084/
- ↑ Baumann M, Bartels F, Finke C, Adamsbaum C, Hacohen Y, Rostásy K, et al. E.U. paediatric MOG consortium consensus: Part 2 – Neuroimaging features of paediatric myelin oligodendrocyte glycoprotein antibody-associated disorders. European Journal of Paediatric Neurology. 2020 Nov 1;29:14–21.
- ↑ Hacohen Y, Rossor T, Mankad K, Chong W ‘Kling,’ Lux A, Wassmer E, et al. ‘Leukodystrophy-like’ phenotype in children with myelin oligodendrocyte glycoprotein antibody-associated disease. Dev Med Child Neurol [Internet]. 2018 Apr 1 [cited 2023 Nov 23];60(4):417–23. Available from: https://onlinelibrary.wiley.com/doi/full/10.1111/dmcn.13649
- ↑ Wendel EM, Baumann M, Barisic N, Blaschek A, Coelho de Oliveira Koch E, Della Marina A, et al. High association of MOG-IgG antibodies in children with bilateral optic neuritis. Eur J Paediatr Neurol [Internet]. 2020 Jul 1 [cited 2023 Oct 31];27:86–93. Available from: https://pubmed.ncbi.nlm.nih.gov/32327391/
- ↑ Jump up to: 20.0 20.1 Song H, Zhou H, Yang M, Tan S, Wang J, Xu Q, et al. Clinical characteristics and prognosis of myelin oligodendrocyte glycoprotein antibody-seropositive paediatric optic neuritis in China. Br J Ophthalmol [Internet]. 2019 Jun 1 [cited 2023 Oct 31];103(6):831–6. Available from: https://pubmed.ncbi.nlm.nih.gov/30049802/
- ↑ Baumann M, Grams A, Djurdjevic T, Wendel EM, Lechner C, Behring B, et al. MRI of the first event in pediatric acquired demyelinating syndromes with antibodies to myelin oligodendrocyte glycoprotein. J Neurol [Internet]. 2018 Apr 1 [cited 2023 Oct 31];265(4):845–55. Available from: https://pubmed.ncbi.nlm.nih.gov/29423614/
- ↑ Ciron J, Cobo-Calvo A, Audoin B, Bourre B, Brassat D, Cohen M, et al. Frequency and characteristics of short versus longitudinally extensive myelitis in adults with MOG antibodies: A retrospective multicentric study. Mult Scler [Internet]. 2020 Jul 1 [cited 2023 Oct 31];26(8):936–44. Available from: https://pubmed.ncbi.nlm.nih.gov/31148523/
- ↑ Tantsis EM, Prelog K, Alper G, Benson L, Gorman M, Lim M, et al. Magnetic resonance imaging in enterovirus-71, myelin oligodendrocyte glycoprotein antibody, aquaporin-4 antibody, and multiple sclerosis-associated myelitis in children. Dev Med Child Neurol [Internet]. 2019 Sep 1 [cited 2023 Oct 31];61(9):1108–16. Available from: https://pubmed.ncbi.nlm.nih.gov/30537075/
- ↑ Jump up to: 24.0 24.1 Klein da Costa B, Banwell BL, Sato DK. Treatment of MOG-IgG associated disease in paediatric patients: A systematic review. Mult Scler Relat Disord [Internet]. 2021 Nov 1 [cited 2023 Oct 31];56. Available from: https://pubmed.ncbi.nlm.nih.gov/34450460/
- ↑ Bonnan M, Valentino R, Debeugny S, Merle H, Fergé JL, Mehdaoui H, et al. Short delay to initiate plasma exchange is the strongest predictor of outcome in severe attacks of NMO spectrum disorders. J Neurol Neurosurg Psychiatry [Internet]. 2018 Apr 1 [cited 2023 Dec 7];89(4):346–51. Available from: https://pubmed.ncbi.nlm.nih.gov/29030418/
- ↑ Chen JJ, Flanagan EP, Pittock SJ, Stern NC, Tisavipat N, Bhatti MT, et al. Visual Outcomes Following Plasma Exchange for Optic Neuritis: An International Multicenter Retrospective Analysis of 395 Optic Neuritis Attacks. Am J Ophthalmol [Internet]. 2023 Aug 1 [cited 2023 Dec 7];252:213–24. Available from: https://pubmed.ncbi.nlm.nih.gov/36822570/
- ↑ Nosadini M, Eyre M, Giacomini T, Valeriani M, Della Corte M, Praticò AD, et al. Early Immunotherapy and Longer Corticosteroid Treatment Are Associated With Lower Risk of Relapsing Disease Course in Pediatric MOGAD. Neurology - Neuroimmunology Neuroinflammation [Internet]. 2023 Jan 1 [cited 2023 Oct 31];10(1). Available from: https://nn.neurology.org/content/10/1/e200065
- ↑ Al-Ani A, Chen JJ, Costello F. Myelin oligodendrocyte glycoprotein antibody-associated disease (MOGAD): current understanding and challenges. J Neurol [Internet]. 2023 Aug 1 [cited 2023 Dec 7];270(8):1. Available from: /pmc/articles/PMC10165591/
- ↑ Jump up to: 29.0 29.1 Bruijstens AL, Wendel EM, Lechner C, Bartels F, Finke C, Breu M, et al. E.U. paediatric MOG consortium consensus: Part 5 – Treatment of paediatric myelin oligodendrocyte glycoprotein antibody-associated disorders. European Journal of Paediatric Neurology. 2020 Nov 1;29:41–53.
- ↑ Bruijstens AL, Breu M, Wendel EM, Wassmer E, Lim M, Neuteboom RF, et al. E.U. paediatric MOG consortium consensus: Part 4 e Outcome of paediatric myelin oligodendrocyte glycoprotein antibody-associated disorders. 2020 [cited 2023 Oct 31]; Available from: https://doi.org/10.1016/j.ejpn.2020.10.007
- ↑ Jump up to: 31.0 31.1 Havla J, Pakeerathan T, Schwake C, Bennett JL, Kleiter I, Felipe-Rucián A, et al. Age-dependent favorable visual recovery despite significant retinal atrophy in pediatric MOGAD: how much retina do you really need to see well? J Neuroinflammation [Internet]. 2021 Dec 1 [cited 2023 Nov 23];18(1). Available from: https://pubmed.ncbi.nlm.nih.gov/34051804/
- ↑ Bruijstens AL, Wendel EM, Lechner C, Bartels F, Finke C, Breu M, et al. E.U. paediatric MOG consortium consensus: Part 5 – Treatment of paediatric myelin oligodendrocyte glycoprotein antibody-associated disorders. European Journal of Paediatric Neurology. 2020 Nov 1;29:41–53.
- ↑ Satukijchai C, Mariano R, Messina S, Sa M, Woodhall MR, Robertson NP, et al. Factors Associated With Relapse and Treatment of Myelin Oligodendrocyte Glycoprotein Antibody–Associated Disease in the United Kingdom. JAMA Netw Open [Internet]. 2022 Jan 4 [cited 2023 Nov 23];5(1):e2142780–e2142780. Available from: https://jamanetwork.com/journals/jamanetworkopen/fullarticle/2787769
- ↑ Chen B, Gomez-Figueroa E, Redenbaugh V, Francis A, Satukijchai C, Wu Y, et al. Do Early Relapses Predict the Risk of Long-Term Relapsing Disease in an Adult and Paediatric Cohort with MOGAD? Ann Neurol [Internet]. 2023 Sep 1 [cited 2023 Nov 23];94(3):508–17. Available from: https://pubmed.ncbi.nlm.nih.gov/37394961/