Pediatric Glaucoma Following Cataract Surgery
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
Introduction
Pediatric glaucoma comprises a group of disorders that result in progressive irreversible optic neuropathy and subsequent vision loss if left untreated.[1] The Childhood Glaucoma Research Network (CGRN) proposed a classification system for distinguishing the etiologies within this group, one of which includes Glaucoma Following Cataract Surgery (GFCS). GFCS is a category diagnosed only after cataract surgery, without a history of trauma, pre-existing congenital eye anomalies, or systemic syndromes. The most common type of GFCS is the open-angle configuration, accounting for 75-94% of cases.[2][3] Angle-closure GFCS was more common prior to advances in automated lensectomy and vitrectomy techniques for cataract extraction. Despite this reduction in complication rate (i.e., pupillary block), GFCS remains one of the most common conditions that can affect vision in the pediatric population, comprising 11.4-41% of all pediatric glaucoma.[1][2][3][4] [5][6]
Pathogenesis
The pathogenesis is thought to be multifactorial; a synergy of mechanical and chemical changes to the outflow system are proposed to occur after cataract surgery. Theories suggest a mechanical loss of support to the trabecular meshwork (TM) after a lensectomy, in addition to the chemical alteration of the TM morphology and gene expression due to inflammatory mediators from the vitreous cavity. [7] Other studies have described post-operative TM obstruction and anterior chamber fibrosis from residual lens material and synechiae formation.[7][8]
Presentation and Incidence
Typically, patients present with elevated intraocular pressure (IOP) following childhood cataract surgery. They may present with vague symptoms of irritability or photophobia but are typically asymptomatic until structural and functional optic nerve damage is detected. Asrani and Wilensky[9] noted that the visual acuity at the time of diagnosis was poor in most patients (worse than 20/200 in 51.6%). Diagnosis relies on clinical examination, as glaucoma could occur at any time, from days to decades after cataract surgery.[10][11] The average interval between lensectomy and diagnosis of glaucoma has been reported to range from 1.5 months up to 19 years depending on the population studied, highlighting the need for long-term follow-up. In children with bilateral cataracts, the median time to postoperative glaucoma diagnosis was longer than in children with unilateral cataract (4.4 months and 1.5 months, respectively) with decreasing age at surgery being the strongest factor independently associated with increased risk in bilateral cataracts (HR 1.05; 95% CI 1.02 to 1.09; P<0.001).[12]
The incidence of GFCS has been reported to range between 6% and 42% of eyes,[4][5][13] with large variation within the literature based on the population being studied. The Infant Aphakia Treatment Study (IATS), a large randomized prospective clinical trial studying GFCS, reported that among 114 infants with unilateral congenital cataract removed at <6 months of age, the risk of glaucoma diagnosis at 1 and 10 years following cataract surgery rose to 22% and 40%, respectively.[13]
While most GFCS have open anterior chamber angles, there may be anatomical changes to the angle or malpositioning of the iris. In a retrospective review conducted by Chen et al.[14], gonioscopy showed open-angles in 139 (93.9%) of the 148 eyes diagnosed with aphakic glaucoma. In a study that was performed using anterior segment ultrasound biomicroscopy on 28 patients who had undergone congenital cataract surgery, Nishijima et al.[15] revealed high iris insertion in 21 eyes, 10 of which developed elevated IOP (47.6%). Walton et al.[16] also noted that in most eyes presenting with GFCS (96% of 65 eyes), the iris had forward positioning inserted into the middle or posterior TM. In a sample of aphakic and pseudophakic eyes following congenital cataract surgery, Balekudaru and colleagues performed gonioscopy and found high iris insertions in 12 eyes. The same study confirmed narrow anterior chamber angles by Shaffer grading in 11 eyes after cataract surgery (10.9%), which resulted in elevated IOP in 2 of these eyes. Out of this subset, one eye developed glaucoma and the other was a glaucoma suspect.[17]
Risk Factors
The risk for GFCS is influenced by the early timing of surgery (i.e., within the first year of life).[3][13][17][10][18][19][20] The risk of glaucoma development in the patients that underwent pediatric cataract surgery before the age of 9 months was found to be 3.8 times more than those who were operated on after the age of 9 months.[21] [20] The 1-year IATS study found a 1.6 times higher risk of glaucoma for each month of age younger at cataract surgery.[22] Although younger age at the time of cataract surgery has been a consistent risk factor across the literature, this is often still pursued early on due to the deleterious effects on visual deprivation and consequent amblyopia.[4]
Anatomic factors such as shorter axial length[9] and congenital anterior segment abnormalities (i.e., microcornea, persistent hyperplastic vitreous, also known as persistent fetal vasculature (PFV)) have been implicated.[5] The 5-year follow-up from IATS identified corneal diameter ≤10 vs. >10 mm as a significant risk factor on multivariate analysis, as a smaller corneal diameter may reflect an abnormal anterior segment.[19] The PFV has been documented in previous studies to be another important predictor of secondary glaucoma. The one-year IATS study results showed that the odds of developing a glaucoma-related adverse event was 3.1 times higher for a child with PFV.[22] Furthermore, Spiess et al. suggested PFV as a predictive factor of time to Ahmed glaucoma valve failure (hazard ratio: 5.77, P = 0.004).[23] However, it should be noted that patients with the presence of PFV-related cataracts have thicker corneas compared to the contralateral normal eyes and those with non-PFV, which may lead to the false diagnosis of ocular hypertension if central corneal thickness (CCT) was not accounted for in IOP measurements.[24]
About 69% of GFCS were bilateral in a study by Bhola et. al[11] as compared with 62% in Chen et al. [14], and 81% in Asrani and Wilensky’s study[9]. Intricacies of the cataract surgery may also influence the risk of GFCS. Studies have found predictive factors to include retained lens proteins.[4][17][14] In a retrospective review conducted by Chen et al.[14], 15% of eyes with GFCS were found to have retained lens material postoperatively; in the cited literature from that study, a reported 41.6% to 78% of eyes diagnosed with GFCS had residual cortex or lens material.[16][14]
Some studies found that primary intraocular lens (IOL) implantation decreases the incidence of GFCS.[4][10][14][5] However, the IATS study revealed that the risk of developing glaucoma after lensectomy is similar for patients with pseudophakic and aphakic conditions at 5 and 10 years post-lensectomy.[13][19] Similarly, after adjustment for age at surgery and axial length, IOL implantation was not associated with the risk of glaucoma for children with either bilateral or unilateral cataract history as reported by Solebo and Rahi[12] or Freedman et. al.[6]
Patients with secondary intraocular procedures performed following cataract surgery can also increase the risk of GFCS.[4][10][19][18] For instance, in the pediatric population, pseudophakic eyes often require additional surgical procedures for the management of complications more frequently than aphakic eyes. According to the IATS, 72% of the patients who had additional intraocular surgeries were pseudophakic, while 16% had aphakic eyes.[19]
Management
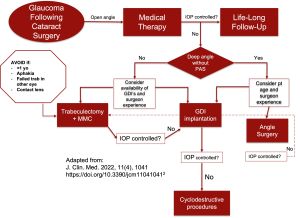
Medical therapy
Medical therapy is the cornerstone of the management of GFCS, unlike primary congenital glaucoma (PCG) which responds inadequately to medical therapy. There are five available studies in which long-term IOP control was achieved with medication alone as the initial treatment in patients with GFCS.[2] The largest study cohorts report success rates of 40%-63%.[11][2]
The choice of medications includes topical beta blockers, prostaglandin analogs, or carbonic anhydrase inhibitors. Lipophillic alpha-adrenergic (i.e., brimonidine) should be avoided in children below the age of 2 and should be used cautiously below the age of 10 years due to their potentially life-threatening side effects, including central nervous system and respiratory depression. Echothiophate iodide 0.125% (EI) is a miotic that has been described in adults with pseudophakic open-angle glaucoma by Schmidt et al. who reported that 20 out of 24 eyes on maximal medical therapy (83.4%) had further sustained reduction of IOP over 11 months.[25] In a retrospective series of 32 pediatric eyes with GFCS, the addition of EI 0.125%, in combination with other medications reduced IOP by about 33% over long-term follow-up.[25]
Surgery
When medical management fails, surgical intervention may be utilized. Out of 35 eyes in a retrospective study of patients with GFCS, fifteen eyes (27%) required one or more surgical procedures to control the IOP during follow-up at a mean interval of 64.6 months between the diagnosis of glaucoma and the first surgical intervention. Of 25 eyes with glaucoma by the 10-year examination in the IATS, 11 eyes underwent glaucoma surgery and 7 (64%) required a single surgery.[13]
Several surgical techniques have been effective at lowering IOP, including angle surgery, trabeculectomy, glaucoma drainage devices (GDDs), endocyclophotocoagulation (ECP), cyclophotocoagulation (CPC), and the more recent minimally invasive glaucoma surgery (MIGS). In a systematic review investigating the management of GFCS by Simons et al.[2] five out of 39 studies described success rates with medication alone, 7 (out of 39) studies examined success rates of angle surgery, 8 studies examined success rates of trabeculectomy, 14 studies examined success rates of GDDs, and 9 out of 39 studies examined success rates of cyclodestructive procedures. However, in general, studies that report treatment outcomes specifically for GFGS are limited and lacking consensus because of large variations in the population studied, length of follow-up, and study design.
Angle surgery
Several studies have demonstrated the efficacy of angle surgery in pediatric GFCS.[26] Bothun et al. reported a 57% success rate with angle surgeries (trabeculotomy 180 or goniotomy) in their retrospective case series consisting of 14 eyes with GFCS.[27] Lyons et. al[28] performed goniotomy in 90 eyes of 61 patients with childhood glaucoma, and 52 eyes (58%) achieved complete success after 1 or 2 goniotomies, 25% of which were eyes with aphakic glaucoma. Bothun and colleagues reported that patients with aphakic glaucoma who received goniotomy as the first-line procedure needed subsequent tube shunting procedures at a higher rate than patients receiving trabeculotomy first, suggesting that trabeculotomy being a more promising initial surgical procedure.[27] In addition, repeat trabeculotomy has higher success rates as the amount of angle treated is extended. In a study comparing the success rates of trabeculotomy surgeries amongst the different types of childhood glaucoma, there was no significant difference in PCG (73%), JOAG (88%), and GFCS (93%).[29]
Circumferential trabeculotomy has been more successful compared to <180° standard trabeculotomy in PCG (33), either by two-site rigid probe or via microcatheter-assisted. El Sayed and colleagues[30] studied the results of the two-site trabeculotomy technique, using the rigid-probe trabeculotomies in pediatric GFCS. They achieved success in 26 eyes (89.6%), of which 15 were controlled without medications. They did not find a significant difference in IOP, medications or success between aphakic and pseudophakic eyes nor between eyes that had 360° trabeculotomy and eyes that had a 180–270° incision.
Lim and colleagues[31] investigated outcomes of 360 catheter trabeculotomy via the iTrack microcatheter (Iscience Interventional, Menlo Park, CA, USA) in a large sample of medically refractory glaucomatous eyes, including 25 GFCS eyes, over an average of 31.5 months. The average pre-operative IOP was 31.5±7.5 mmHg on an average of three drops pre-operatively. Post-operative IOP subsequently lowered to 19.7 mmHg (P < 0.001), and patients were on an average of 2.4 drops postoperatively (P= 0.015). These data suggest that microcatheter-assisted 360° trabeculotomy is a safe and effective initial surgical procedure for management of GFCS.
Trabeculectomy
A trabeculectomy offers poor surgical success rates in GFCS. Madal and colleagues performed the largest retrospective series of trabeculectomy both with and without Mitomycin C (0.4 mg/ml) (MMC) performed in 23 Asian Indian aphakic (21) and pseudophakic (2) eyes. Complete success (IOP between 6 and 21 mmHg without medication, further surgery nor sight-threatening complications) was achieved in only 37% after a mean follow-up of 2 years.[32]
Factors that impede the success of trabeculectomy in children include vigorous wound-healing responses due to thicker Tenon’s capsules and conjunctiva, predisposing to subconjunctival fibrosis and bleb failure. The use of anti-fibroproliferative agents such as 5-fluorouracil (5-FU) and MMC may improve the surgical prognosis, however, the study by Mandal et. al[32] found no difference between the groups with or without MMC in GFCS eyes. With a similar definition of success by Azuara-Blanco et al, all trabeculectomies with MMC failed in eight eyes with aphakic glaucoma after a mean of 19 months.[33]
Glaucoma drainage device
Amongst the earliest valve implants was the Molteno implant which investigators have reported with positive success rates.[34][35] Molteno et al.[35] and Billson et al.[34] reported success rates of 87 and 95%, respectively. Billson and colleagues achieved IOP control with a follow-up of 1-7 years in 8 of 11 eyes with childhood aphakic glaucoma after a two-stage Molteno implant (success defined as 21 mmHg without medication).[34]
The Ahmed Glaucoma Valve (AGV; New World Medical) and the Baerveldt Glaucoma Implant (BGI; Johnson & Johnson Vision), have been more commonly utilized as primary or secondary surgery in GFCS.[36] Esfandiari et al. retrospectively evaluated outcomes of both AGV and BGI in 28 eyes with GFCS in patients with a mean age of 4.2 years at the time of surgery. They found an average IOP reduction of 45% at 4 years. In addition, they reported a cumulative probability of failure (IOP < 5 mmHg or > 21 mmHg or not reduced by 20% below baseline on 2 consecutive follow-up visits) of 28% at 3 and 4 years postoperatively, AGV and BGI respectively.[37] The AGV has specifically been studied in a study of 29 eyes by Spiess et al., with 41% with PFV and 59% with non-PFV. This surgery had success rates of 37.5% and 88.2% in year 1 and 28.1% and 71.9% in year 5, respectively.[23]
In a retrospective study, Parkavan et al. compared 15 eyes that underwent MMC-augmented trabeculectomy to MMC-augmented AGV in patients less than 16 years of age with GFCS.[38] Complete success rates were 33.3% in the MMC-augmented trabeculectomy group versus 20% in the MMC-augmented AGV group. Although these authors report no statistically significant difference in the success rates or complication rates between the treatment groups, the literature suggests that GDDs have a higher success rate than trabeculectomy in the management of GFCS with a significantly better safety profile. A trabeculectomy is often avoided as it may preclude the use of aphakic contact lenses in the case of a bleb and begets bleb-related complications such as infection, leak, or over-filtration.[2]
Cyclophotocoagulation
The initial success rates of CPC may vary from 37% to 74% and can be repeated for further IOP reduction during the first year after surgery.[39][40] After a follow-up of 7.2 years, a cohort study had a success rate of 54% out of 35 aphakic or pseudophakic GFCS eyes. Another study evaluated transscleral diode CPC in aphakic and phakic eyes with refractory glaucoma and compared the rates of those achieving IOP < 22 mmHg or reduction by 30%, with and without antiglaucoma medications. After one treatment session, 62% had a clinically useful reduction in IOP (<22 mmHg or by 30%), but this had fallen to 37% by 12 months. With repeat cyclodiode, 72% had a clinically useful reduction in IOP for a year or more. He reported that aphakic patients had a more sustained IOP control achieved at 42% at one year versus 14% in phakic eyes.[41]
The ECP has been utilized in the treatment of GFGS. Carter et al. conducted 180-360 degree treatments in patients with GFCS over a mean follow-up time of 44 months.[42] They found an overall success rate (IOP ≤ 24 mmHg and IOP decrease > 15% despite adding topical therapy) of 53% (18/34). Thirteen of the 34 eyes (38%) received one treatment only and were deemed a success. Glaser et al. found a success rate of 64% at 1 year, 36% at 3 years, and 16% at 5 years after a single treatment (30), with success rates, increase with more than one treatment.[43]
Minimally invasive glaucoma surgery
MIGS in childhood glaucoma has been of growing interest in the recent decade. The Kahook dual-blade (KDB, New World Medical, Rancho Cucamonga, CA) is a device that advances along the iridotrabecular angle with 2 sharp parallel blades to excise the TM strip, rather than incising it such as in conventional goniotomy. Khouri and Wong published a report of two eyes with GFCS who underwent KDB and at a follow-up of 7-10 weeks had substantial reduction in IOP from 35 to 17 mmHg in the right eye and from 52 to 18 mmHg in the left eye after 7 to 10 weeks.[44]
The gonioscopy assisted trans luminal trabeculotomy (GATT) is a technique involving an illuminated microcatheter or suture advancing circumferentially through Schlemm’s canal and pulled out, creating an ab-interno circumferential trabeculotomy.[45] Quan et. al investigated GATT in 13 eyes with GFCS and reported a success rate of 38.5%. Successful circumferential angle treatment was achieved in 11 eyes (84.6%).[46] The Trab360 (Sight Sciences, Menlo Park, CA, USA) is another method of achieving circumferential trabeculotomy in childhood glaucoma with a novel retractable suture. Areaux et al. reported outcomes of the Trab360 device in 46 eyes of 41 patients with childhood glaucoma (5 eyes with GFCS) with success rates as ≤ 24 mmHg without medication at a mean follow-up time of 14.5 months. The success rate was reported 83.3% overall, and 60% of eyes with GFCS.[47]
Ruparelia et. al describe a case of a 10-year old boy with aphakic glaucoma. The IOP at presentation was 31 mmHg with maximal tolerated medical therapy. After a tube shunt, revision, and subsequent removal, two treatments of micro-pulse transscleral laser therapy, the patient underwent XEN Gel Stent implantation with MMC ab-externo. After conjunctival dehiscence, the XEN Gel was explanted. A second ab-interno XEN Gel was placed and six months later, IOP reduced to 6 to 8 mmHg without anti-glaucoma medications.[48]
Conclusion
The GFCS is one of the 7 entities of childhood glaucoma based on the CGRN classification. Despite the reduction in the complication rate following cataract surgery from advances in automated lensectomy and vitrectomy techniques, GFCS remains one of the most common conditions that can affect vision in the pediatric population, comprising 11-41% of all pediatric glaucoma. Unlike in other forms of childhood glaucoma, GFCS responds well to medical therapy. Surgical intervention has evolved and provides good outcomes in medically-uncontrolled glaucoma cases.
References
- ↑ 1.0 1.1 Tam EK, Elhusseiny AM, Shah AS, Mantagos IS, VanderVeen DK. Etiology and outcomes of childhood glaucoma at a tertiary referral center. J AAPOS. 2022;26(3):117.e1-117.e6. doi:10.1016/j.jaapos.2021.12.009
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 Simons AS, Casteels I, Grigg J, Stalmans I, Vandewalle E, Lemmens S. Management of Childhood Glaucoma Following Cataract Surgery. J Clin Med. 2022 Feb 17;11(4):1041. doi: 10.3390/jcm11041041.
- ↑ 3.0 3.1 3.2 Kirwan C, O'Keefe M. Paediatric aphakic glaucoma. Acta Ophthalmol Scand. 2006;84(6):734-739. doi:10.1111/j.1600-0420.2006.00733.x
- ↑ 4.0 4.1 4.2 4.3 4.4 4.5 Lawrence MG, Kramarevsky NY, Christiansen SP, Wright MM, Young TL, Summers CG. Glaucoma following cataract surgery in children: surgically modifiable risk factors. Trans Am Ophthalmol Soc. 2005;103:46-55.
- ↑ 5.0 5.1 5.2 5.3 Swamy BN, Billson F, Martin F, et al. Secondary glaucoma after paediatric cataract surgery. British Journal of Ophthalmology. 2007;91(12):1627-1630.
- ↑ 6.0 6.1 Freedman SF, Kraker RT, Repka MX, Wallace DK, de Alba Campomanes A, Yanovitch TL, Orge FH, Gearinger MD; Pediatric Eye Disease Investigator Group (PEDIG). Incidence and Management of Glaucoma or Glaucoma Suspect in the First Year After Pediatric Lensectomy. JAMA Ophthalmol. 2020 Jan 1;138(1):71-75. doi: 10.1001/jamaophthalmol.2019.4571.
- ↑ 7.0 7.1 Yeung HH, Kumar-Singh R, Walton DS. Infantile Aphakic Glaucoma: A Proposed Mechanism. Journal of Pediatric Ophthalmology & Strabismus. 2022 Jul 1;59(4):236-42.
- ↑ Gouda J, Tomairek RH, Elhusseiny AM, El-Fayoumi D, Awadein A, Gawdat G, Elhilali H. Changes in Intraocular Pressure and Anterior Chamber Angle After Congenital Cataract Extraction. J Glaucoma. 2021 Jan 1;30(1):61-64. doi: 10.1097/IJG.0000000000001681.
- ↑ 9.0 9.1 9.2 Asrani SG, Wilensky JT. Glaucoma after congenital cataract surgery. Ophthalmology. 1995;102(6):863-867. doi:10.1016/s0161-6420(95)30942-6
- ↑ 10.0 10.1 10.2 10.3 Sahin A, Caça I, Cingü AK, Türkcü FM, Yüksel H, Sahin M, Cinar Y, Ari S. Secondary glaucoma after pediatric cataract surgery. Int J Ophthalmol. 2013 Apr 18;6(2):216-20. doi: 10.3980/j.issn.2222-3959.2013.02.21.
- ↑ 11.0 11.1 11.2 Bhola R, Keech RV, Olson RJ, Petersen DB. Long-term outcome of pediatric aphakic glaucoma. J AAPOS. 2006;10(3):243-248.
- ↑ 12.0 12.1 Solebo AL, Rahi JS; British Congenital Cataract Interest Group. Glaucoma following cataract surgery in the first 2 years of life: frequency, risk factors and outcomes from IoLunder2. Br J Ophthalmol. 2020;104(7):967-973.
- ↑ 13.0 13.1 13.2 13.3 13.4 Freedman SF, Beck AD, Nizam A, Vanderveen DK, Plager DA, Morrison DG, Drews-Botsch CD, Lambert SR; Infant Aphakia Treatment Study Group. Glaucoma-Related Adverse Events at 10 Years in the Infant Aphakia Treatment Study: A Secondary Analysis of a Randomized Clinical Trial. JAMA Ophthalmol. 2021 Feb 1;139(2):165-173. doi: 10.1001/jamaophthalmol.2020.5664.
- ↑ 14.0 14.1 14.2 14.3 14.4 14.5 Chen TC, Walton DS, Bhatia LS. Aphakic Glaucoma After Congenital Cataract Surgery. ArchOphthalmol. 2004;122(12):1819–1825. doi:10.1001/archopht.122.12.1819
- ↑ Nishijima K, Takahashi K, Yamakawa R. Ultrasound biomicroscopy of the anterior segment after congenital cataract surgery. Am J Ophthalmol. 2000;130:483–89. doi: 10.1016/S0002-9394(00)00524-9.
- ↑ 16.0 16.1 Walton DS. Pediatric aphakic glaucoma: a study of 65 patients. Trans Am Ophthalmol Soc 1995; 93:403-420.
- ↑ 17.0 17.1 17.2 Balekudaru S, Agarkar S, Guha S, et al. Prospective analysis of the predictors of glaucoma following surgery for congenital and infantile cataract. Eye. 2018;33(5):796-803. doi:10.1038/s41433-018-0316-8
- ↑ 18.0 18.1 Mataftsi A, Haidich A, Kokkali S, et al. Postoperative Glaucoma Following Infantile Cataract Surgery: An Individual Patient Data Meta-analysis. JAMA Ophthalmol. 2014;132(9):1059–1067. doi:10.1001/jamaophthalmol.2014.1042
- ↑ 19.0 19.1 19.2 19.3 19.4 Freedman SF, Lynn MJ, Beck AD, et al. Glaucoma-Related Adverse Events in the First 5 Years After Unilateral Cataract Removal in the Infant Aphakia Treatment Study. JAMA Ophthalmol. 2015;133(8):907-914.
- ↑ 20.0 20.1 Rabiah PK. Frequency and predictors of glaucoma after pediatric cataract surgery. Am J Ophthalmol. 2004;137(1):30-37. doi:10.1016/s0002-9394(03)00871-7
- ↑ Abdelmassih Y, Beaujeux P, Dureau P, Edelson C, Caputo G. Incidence and Risk Factors of Glaucoma Following Pediatric Cataract Surgery With Primary Implantation. Am J Ophthalmol. 2021;224:1-6. doi:10.1016/j.ajo.2020.09.025
- ↑ 22.0 22.1 Beck AD, Freedman SF, Lynn MJ, Bothun E, Neely DE, Lambert SR; Infant Aphakia Treatment Study Group. Glaucoma-related adverse events in the Infant Aphakia Treatment Study: 1-year results. Arch Ophthalmol. 2012 Mar;130(3):300-5. doi: 10.1001/archophthalmol.2011.347.
- ↑ 23.0 23.1 Spiess K, Peralta Calvo J. Outcomes of Ahmed glaucoma valve in paediatric glaucoma following congenital cataract surgery in persistent foetal vasculature. Eur J Ophthalmol. 2021;31(3):1070-1078. doi:10.1177/1120672120919066
- ↑ Elhusseiny AM, Gouda J, Farag C, Chauhan MZ, Arfeen SA, Elhilali HM. Central corneal thickness profile in relation to pediatric cataract morphology. J AAPOS. 2022;S1091-8531(22)00422-0. doi:10.1016/j.jaapos.2022.07.003
- ↑ 25.0 25.1 Schmidt KG, Horowitz Y, Buckman G, Segev E, Levinger E, Geyer O. Lowering of IOP by echothiophate iodide in pseudophakic eyes with glaucoma. Curr Eye Res. 2010 Aug;35(8):698-702. doi: 10.3109/02713681003794076.
- ↑ Jamerson EC, Solyman O, Yacoub MS, Abushanab MMI, Elhusseiny AM. Angle Surgery in Pediatric Glaucoma Following Cataract Surgery. Vision (Basel). 2021;5(1):9. Published 2021 Feb 5. doi:10.3390/vision5010009
- ↑ 27.0 27.1 Bothun E.D., Guo Y., Christiansen S.P., Summers C.G., Anderson J.S., Wright M.M., Kramarevsky N.Y., Lawrence M.G. Outcome of angle surgery in children with aphakic glaucoma. J. AAPOS. 2010;14:235–239. doi: 10.1016/j.jaapos.2010.01.005
- ↑ CHRISTOPHER John LYONS, Sharon Armarnik, Stephen Farrell; Goniotomy for the childhood glaucomas: Where is it most useful? Sharon Armarnik, Stephen Farrell, Christopher Lyons University of British Columbia and BC Children’s Hospital. Invest. Ophthalmol. Vis. Sci. 2019;60(9):6648.
- ↑ Rojas C, Bohnsack BL. Rate of Complete Catheterization of Schlemm's Canal and Trabeculotomy Success in Primary and Secondary Childhood Glaucomas. Am J Ophthalmol. 2020;212:69-78. doi:10.1016/j.ajo.2019.11.029
- ↑ El Sayed, Y.M., Elhusseiny, A.M., Gawdat, G.I. et al. One-year results of two-site trabeculotomy in paediatric glaucoma following cataract surgery. Eye 35, 1637–1643 (2021). https://doi.org/10.1038/s41433-020-01138-w
- ↑ Lim ME, Dao JB, Freedman SF. 360-Degree Trabeculotomy for Medically Refractory Glaucoma Following Cataract Surgery and Juvenile Open-Angle Glaucoma. Am J Ophthalmol. 2017;175:1-7. doi:10.1016/j.ajo.2016.11.011
- ↑ 32.0 32.1 Mandal AK, Bagga H, Nutheti R, Gothwal VK, Nanda AK. Trabeculectomy with or without mitomycin-C for paediatric glaucoma in aphakia and pseudophakia following congenital cataract surgery. Eye (Lond). 2003;17(1):53-62. doi:10.1038/sj.eye.6700180
- ↑ Azuara-Blanco, Augusto, et al. "Filtration procedures supplemented with mitomycin C in the management of childhood glaucoma." British Journal of Ophthalmology 83.2 (1999): 151-156.
- ↑ 34.0 34.1 34.2 Billson F, Thomas R, Alward W. The use of two-stage Molteno implants in developmental glaucoma. J Pediatr Ophthalmol Strabismus 1989; 26: 3–8
- ↑ 35.0 35.1 Molteno ACB, Ancker E, Biljon GV. Surgical technique for advanced juvenile glaucoma. Arch Ophthalmol 1984; 102: 51–57.
- ↑ Elhusseiny AM, VanderVeen DK. Outcomes of Glaucoma Drainage Devices in Childhood Glaucoma. Semin Ophthalmol. 2020 Apr 2;35(3):194-204. doi: 10.1080/08820538.2020.1781906. Epub 2020 Jun 20.
- ↑ Esfandiari H, Kurup SP, Torkian P, Mets MB, Rahmani B, Tanna AP. Long-term clinical outcomes of ahmed and baerveldt drainage device surgery for pediatric glaucoma following cataract surgery. J Glaucoma. 2019;28(10):865–870. doi:10.1097/IJG.0000000000001335.
- ↑ Pakravan M, Homayoon N, Shahin Y, Ali Reza BR. Trabeculectomy with mitomycin c versus ahmed glaucoma implant with mitomycin c for treatment of pediatric aphakic glaucoma. J Glaucoma. 2007;16 (7):631–636.
- ↑ Schlote T, Derse M, Rassmann K, et al. Efficacy and safety of contact transscleral diode laser cyclophotocoagulation for advanced glaucoma. J Glaucoma. 2001;10:294-301.
- ↑ Cantor A.J., Wang J., Li S., Neely D.E., Plager D.A. Long-term efficacy of endoscopic cyclophotocoagulation in the management of glaucoma following cataract surgery in children. J. Am. Assoc. Pediatr. Ophthalmol. Strabismus. 2018;22:188–191.
- ↑ Kirwan J.F., Shah P., Khaw P.T. Diode laser cyclophotocoagulation: Role in the management of refractory pediatric glaucomas. Ophthalmology. 2002;109:316–323. doi: 10.1016/S0161-6420(01)00898-40.
- ↑ Carter B.C., Plager D.A., Neely D.E., Sprunger D.T., Sondhi N., Roberts G.J. Endoscopic diode laser cyclophotocoagulation in the management of aphakic and pseudophakic glaucoma in children. J. Am. Assoc. Pediatr. Ophthalmol. Strabismus. 2006;11:34–40. doi: 10.1016/j.jaapos.2006.08.015.
- ↑ Glaser T.S., Mulvihill M.S., Freedman S.F. Endoscopic cyclophotocoagulation (ECP) for childhood glaucoma: A large single-center cohort experience. J. Am. Assoc. Pediatr. Ophthalmol. Strabismus. 2019;23:84.e1–84.e7. doi: 10.1016/j.jaapos.2018.10.014
- ↑ Khouri AS, Wong SH. Ab Interno Trabeculectomy With a Dual Blade: Surgical Technique for Childhood Glaucoma. J Glaucoma. 2017;26(8):749-751.
- ↑ Aboalazayem F, Elhusseiny AM, El Sayed YM. Gonioscopy-Assisted Transluminal Trabeculotomy: A Review. Curr Eye Res. 2022 Jun 13:1-10. doi: 10.1080/02713683.2022.2084113.
- ↑ Quan AV, Chen J, Wang YE, et al. Factors Associated With Gonioscopy-Assisted Transluminal Trabeculotomy (GATT) Complications and Failure in Children. Am J Ophthalmol. 2022;241:168-178. doi:10.1016/j.ajo.2022.04.023
- ↑ Areaux RG Jr, Grajewski AL, Balasubramaniam S, et al. Trabeculotomy Ab Interno With the Trab360 Device for Childhood Glaucomas. Am J Ophthalmol. 2020;209:178-186.
- ↑ Ruparelia S, Berco E, Lichtinger A, Shoham-Hazon N. Multiple XEN Gel Stents for Refractory Pediatric Glaucoma. J Pediatr Ophthalmol Strabismus. 2022;59(1):e11-e14. doi:10.3928/01913913-20211101-03