Paul Glaucoma Implant
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
Background
Over the past decade, the use of glaucoma drainage devices (GDDs) has significantly increased in the management of glaucoma. GDDs effectively reduce the intraocular pressure (IOP), however, several complications such as hypotony, endothelial cell loss, and tube erosions are concerning. The two most commonly used GDDs are the Ahmed glaucoma valve (New World Medical, Rancho Cucamonga, CA) and the Baerveldt glaucoma implant (Johnson & Johnson, Santa Ana, CA). The Paul glaucoma implant (PGI, Advanced Ophthalmic Innovations, Singapore, Republic of Singapore) is a novel valveless GDD that was designed by Professor Paul Chew in an attempt to optimize the efficacy with a theoretically higher safety profile. However, literature evaluating its efficacy and safety is still limited.
Device
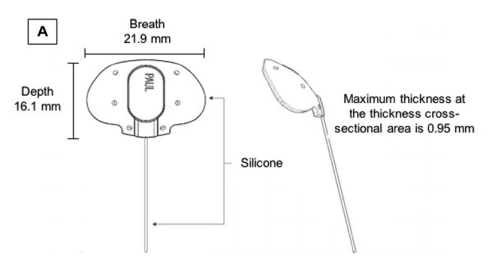
The PGI design has many characteristic features (Figure 1). It is a valveless GDD made up of medical-grade silicone which allows the device pliability and easier implantation. The internal (0.127 mm) and external (0.467 mm) tube diameters are significantly smaller than those of the Ahmed glaucoma valve or Baerveldt glaucoma implant. The smaller diameters reduce the area of contact between the tube and the corneal endothelium which theoretically reduces the rate of endothelial cell loss. The extraocular portion of the tube shunt is smaller which presumably reduces the long-term risk of tube erosion and exposure. Although the tube caliber is smaller than other GDDs, there was no resistance to aqueous flow and theoretically reduces the risk of early hypotony. The lumen can be easily occluded using a 6/0 or 7/0 polypropylene stent compared to the 3/0 which is usually used to occlude the tube in the Baerveldt glaucoma implant.[1] The end plate has been designed to allow more area for aqueous drainage while reducing the area tucked under the recti muscles. The breadth of the endplate is 21.9 mm, and the width is 16.11 mm. The end plate surface area is 342.1 mm2 which is slightly smaller than that of the Baerveldt glaucoma implant but significantly larger than that of the Ahmed glaucoma valve. The anteroposterior depth of the PGI is larger compared to that of the Baerveldt glaucoma implant which allows the plate to extend further back, while the shorter breadth (wingspan) reduces the area of the plate tucked under the recti muscles which may theoretically reduce the postoperative risk of strabismus and diplopia.[1]
Outcomes
A- Adult Glaucoma
The safety and efficacy of the PGI in glaucoma management were first evaluated prospectively by Koh and colleagues[1] in a multicenter, single-arm study at 6 ophthalmology centers. They enrolled 82 patients, of which 74 had a minimum of 1-year follow-up. The primary outcome of the study was the failure rate which was defined as postoperative IOP <6 mmHg or >21 mmHg or IOP reduction of <20% from baseline values for 2 consecutive visits after ≥ 3 months. Loss of vision, need for device removal, or need for further glaucoma surgery was considered a failure. The authors reported a failure rate of 5.4% at 12 months. There was a statistically significant reduction in the mean IOP from 23.1±8.2 mmHg preoperatively to 13.2±3.3 mmHg at the 12-month follow-up visit. There was a statistically significant reduction in the mean number of glaucoma medications from 3.3±0.9 preoperatively to 0.3±0.6 at the 12-month follow-up visit. The most common significant postoperative complications were shallow anterior chamber (11 eyes), hypotony requiring intervention (7 eyes), tube occlusion (5 eyes), and tube exposure (3 eyes). One eye developed postoperative endophthalmitis.[1]
A retrospective study was conducted by Vallabh and colleagues[2] including 99 glaucoma eyes who underwent the PGI surgery and had a minimum of 6 months of follow-up. The authors reported a failure rate, defined as postoperative IOP of >21 mmHg, <20% IOP reduction from baseline values, loss of light perception, need for further glaucoma surgeries, or PGI removal, of 9.3%. There was a statistically significant reduction in the mean IOP from 28.1±9.0 mmHg preoperatively to 13.6±4.7 mmHg at 6 months and 13.3±4.4 mmHg at 12 months (52 out of 97 patients). The mean number of glaucoma medications was significantly reduced from 3.61±1.09 at baseline to 1.22±1.21 at 6 months and 1.25±1.28 at the 12-month follow-up visit (52 patients only). The most common early postoperative complication (≤3 months after surgery) was hyphema (4 eyes), followed by hypotony (2 eyes), and cystoid macular edema (2 eyes).
Another retrospective study by Jose and colleagues[3] included 24 patients at a mean age of 42 years at the time of PGI surgery. All patients had a minimum of 12 month follow-up period. They defined success as a postoperative IOP of >5 mmHg and ≤18 mmHg with at least 30% IOP reduction with (qualified) or without (absolute) glaucoma medications. They reported an absolute success rate of 33% and a qualified success rate of 75%. There was a significant reduction of mean IOP from 31.4±10.0 mmHg preoperatively to 12.5±4.3 mmHg at the last follow-up. The mean number of glaucoma medications was significantly reduced from a mean of 3.0 at the baseline line to 0.9 at the 12-month follow-up. The authors reported no cases of postoperative hypotony requiring intervention.
Tan and colleagues[4] retrospectively evaluated the 2-year outcomes of PGI in 45 patients with a mean follow-up of 24.9±2.0 months. They reported a significant reduction in the mean IOP from 19.8±6.3 mmHg on a mean of 3.2±0.8 glaucoma medications preoperatively to a mean of 13.9±3.7 mmHg on a mean of 0.29±0.65 glaucoma medications at the 24-month follow-up visit. Ten eyes developed shallow anterior chamber postoperatively which were self-limiting and four eyes developed clinically significant hypotony that required intervention.[4]
B- Childhood glaucoma
Limited literature is currently available about the effectiveness and safety of PGI in childhood glaucoma. A small series by Elhusseiny and colleagues[5] evaluated the effectiveness of PGI in 3 eyes with refractory childhood glaucoma with a 9-month follow-up period. They reported a significant reduction of the IOP (<15 mmHg in all 3 eyes at the last follow-up) with no significant intra- or post-operative complications. A larger series by Vallabh and colleagues[6] retrospectively evaluated 25 pediatric glaucoma eyes who underwent PGI surgery. They reported a significant reduction in the mean IOP from 30.9±5.9 mmHg preoperatively to a mean of 13.2±4.9 mmHg at 12 months and 11.8±4.6 mmHg at 24 months with a significant reduction in the number of glaucoma medications after PGI surgery. Qualified success rate, defined as postoperative IOP of 6-21 mmHg and IOP reduction of at least 20% from baseline with or without glaucoma medications, was reported to be 84% at the final follow-up. Eleven eyes (48%) achieved success without the need of any postoperative glaucoma medications.
Conclusions
The PGI is a novel non-valved GDD that effectively reduces the IOP. However, further prospective studies with longer-term follow-up comparing its efficacy and safety to other available GDDs are needed.
References
- ↑ 1.0 1.1 1.2 1.3 Koh V, Chew P, Triolo G, Lim KS, Barton K, Koh V, et al. Treatment Outcomes Using the PAUL Glaucoma Implant to Control Intraocular Pressure in Eyes with Refractory Glaucoma. Ophthalmology Glaucoma. 2020;3(5):350-9
- ↑ Vallabh NA, Mason F, Yu JTS, Yau K, Fenerty CH, Mercieca K, et al. Surgical technique, perioperative management and early outcome data of the PAUL® glaucoma drainage device. Eye. 2021
- ↑ José P, Barão RC, Teixeira FJ, Marques RE, Peschiera R, Barata A, et al. One-Year Efficacy and Safety of the PAUL Glaucoma Implant Using a Standardized Surgical Protocol. J Glaucoma. 2022;31(3):201
- ↑ 4.0 4.1 Tan MCJ, Choy HYC, Koh Teck Chang V, Aquino MC, Sng CCA, Lim DKA, et al. Two-Year Outcomes of the Paul Glaucoma Implant for Treatment of Glaucoma. Journal of Glaucoma. 2022;31(6).
- ↑ Elhusseiny AM, Khodeiry MM, Lee RK, Shaarawy T, Waqar S, Sayed MS. Early Experience with the Paul Glaucoma Implant in Childhood Glaucoma: A Case Series. Clin Ophthalmol. 2023 Jul 6;17:1939-1944. doi: 10.2147/OPTH.S414183.
- ↑ Vallabh NA, Mohindra R, Drysdale E, Mason F, Fenerty CH, Yau K. The PAUL® glaucoma implant: 1-year results of a novel glaucoma drainage device in a paediatric cohort. Graefes Arch Clin Exp Ophthalmol. 2023 Mar 21:1–8.