Parinaud Syndrome
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
Disease
Parinaud syndrome is a relatively uncommon neuroophthalmologic syndrome named for French ophthalmologist, Henri Parinaud. Parinaud syndrome has been known by many different names including dorsal midbrain syndrome, Sylvian aqueduct syndrome, pretectal syndrome, and Koerber-Salus-Elschnig syndrome.[1][2] Parinaud syndrome is defined as a constellation of upward gaze palsy, convergence retraction nystagmus, light-near dissociation, and bilateral lid retraction.[1][3][4][5]
Etiology
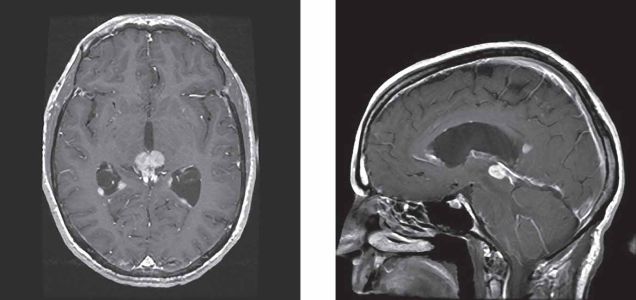
Parinaud syndrome (PS) results from lesions affecting structures in the dorsal midbrain[1][2][6] (e.g., infarction, hemorrhage, tumors, demyelination, inflammation, infection, trauma, hydrocephalus, and arteriovenous malformations). However, the most common causes are pineal region tumors, brainstem hemorrhage, and ischemic infarction.[1][4][7] Pineocytoma and germinoma account for up to 40% of all the tumors causing PS.[1][8] The etiology of PS is also correlated with patient age, with vascular causes being more common among elderly populations and neoplastic causes being more common in younger populations.[6]
Pathophysiology
Involvement of the structures of the dorsal midbrain produce the PS findings. Up gaze palsy is due to damage of the vertical gaze center (rostral interstitial nucleus of the medial longitudinal fasciculus (riMLF) and the interstitial nucleus of Cajal (INC)) and its connections. The riMLF is primarily responsible for vertical saccades, while the INC controls all other vertical eye movements including vertical gaze holding and skew deviation. Both of these nuclei lie in close proximity to the cerebral aqueduct and decussate in the posterior commissure. Their presence in the posterior commissure makes them particularly susceptible to unilateral space occupying lesions and elevated cerebrospinal fluid pressures. Downgaze is relatively preserved in PS until late in the disease due to its bilateral innervation and is typically only affected by large space occupying lesions or bilateral lesions.[9][10][11]
Convergence retraction nystagmus is postulated to be due to damage of the midbrain supranuclear fibers.[9][10][11] These fibers normally exert an inhibitory effect on the third nerve nucleus, thereby preventing activation of the extraocular muscles innervated by cranial nerve three.[9][10] Thus, with the loss of their inhibitory effect, the superior and inferior rectus muscles receive constant stimulation, resulting in retraction of the globe. Additionally, stimulation of the medial rectus muscles causes it to overpower the lateral rectus, leading to involuntary convergence.[12]
Light-near dissociation is due to damage of the pretectal and Edinger-Westphal nuclei, or the decussating fibers of the pretectal nucleus in the posterior commissure.[9][10] Damage to these nuclei results in loss of parasympathetic innervation to the iris sphincter muscles and an inability to constrict the pupil. The nuclei responsible for the pupillary light reflex are thought to lie more dorsally than those controlling the near reflex, making them more susceptible to compressive effects. Therefore, dorsal midbrain lesions typically result in the loss of the pupillary light reflex and, preservation of the near reflex.[9][10][11]
Bilateral lid retraction (Collier sign), like convergence retraction nystagmus, is thought to be due to loss of the supranuclear input to the third nerve nucleus. Without the inhibitory effect of the supranuclear fibers, the levator palpebrae superioris receives constant stimulation via the oculomotor nerve, resulting in lid retraction.[9][10][11]
Presentation
Up gaze palsy is one of the most common initial presenting complaints in patients with PS and is present in 87-100% of patients.[1][7] Limited upward gaze often leads to a preference for downward gaze in primary position and is described as the setting-sun sign.[2][11] Convergence-retraction nystagmus is characterized by irregular, oscillatory movements of the eyes, particularly with upward gaze and saccades upward.[12] Involuntary convergence and globe retraction are also most evident on upward gaze. Approximately 35-88% of patients will experience convergence-retraction nystagmus.[1][7] An example of convergence-retraction nystagmus can be seen at the following link.
https://www.youtube.com/watch?v=B8DPmIZpVm8
Light-near dissociation presents with poor bilateral pupillary constriction in response to light, but preserved constriction with convergence. It is seen in about 65-96% of patients with Parinaud syndrome.[1][7] https://www.aao.org/annual-meeting-video/what-to-make-of-this-neuroophthalmic-sign-eyelid-r
An example of light-near dissociation can be seen at the following link.
https://www.youtube.com/watch?v=mFGO3vzHlG8
Collier’s sign, also known as bilateral lid retraction is seen in around 20-40% of patients.[1][7] The full PS triad of up gaze palsy, convergence retraction nystagmus, and light-near dissociation may only be seen in up to 65% of cases.[1]
In addition to the aforementioned findings, patients may present with the following signs and symptoms:[1][2][4][5][7]
Signs:
- Ataxia
- Exotropia
- Convergence insufficiency
- Papilledema
Symptoms:
- Diplopia
- Blurry vision
- Oscillopsia
- Nausea
- Vomiting
Evaluation
Evaluation of patients with PS begins with a thorough history and physical examination. It is recommended that patients receive a complete eye exam that includes visual acuity determination, pupillary exam, fundoscopy and visual field testing. Neuroimaging with MRI is suggested for all patients with symptoms suggestive of PS.[13] https://www.aao.org/image/dorsal-midbrain-parinaud-syndrome-2
Further testing should revolve around the suspected etiology, such as infectious antibody screening (e.g., syphilis), cerebrospinal fluid analysis, and nerve conduction studies.
Treatment and Outcomes
Treatment involves addressing the underlying condition. However, symptom resolution depends heavily on the precipitating cause. For example, correction of hydrocephalus with CSF shunting generally leads to a complete resolution of all symptoms.[2][14] However, resolution of symptoms following tumor resection is generally incomplete, with only around 12% of patients experiencing complete resolution.[5] Even with treatment, most patients continue to experience at least some residual ocular motility disturbances.[3][4]
Persistent symptoms are first managed conservatively with visual tracking exercises, refractive error correction, prisms, and temporary occlusion.[1][11] Surgical correction may be considered for refractory symptoms. Preferred surgical management includes bilateral inferior rectus recession or superior transposition of the medial and lateral rectus insertions.[1][15] Both methods result in comparable improvement of up gaze palsy and convergence retraction nystagmus.[15]
Resources
https://www.youtube.com/watch?v=c32F374ZiGI
References
- ↑ Jump up to: 1.00 1.01 1.02 1.03 1.04 1.05 1.06 1.07 1.08 1.09 1.10 1.11 1.12 Shields M, Sinkar S, Chan W, Crompton J. Parinaud syndrome: a 25‐year (1991–2016) review of 40 consecutive adult cases. Acta ophthalmologica. 2017 Dec;95(8):e792-3.
- ↑ Jump up to: 2.0 2.1 2.2 2.3 2.4 Chattha AS, Delong GR. Sylvian aqueduct syndrome as a sign of acute obstructive hydrocephalus in children. Journal of Neurology, Neurosurgery & Psychiatry. 1975 Mar 1;38(3):288-96.
- ↑ Jump up to: 3.0 3.1 Goldenberg-Cohen N, Haber J, Ron Y, Kornreich L, Toledano H, Snir M, Cohen IJ, Michowiz S. Long-term ophthalmological follow-up of children with Parinaud syndrome. Ophthalmic Surgery, Lasers and Imaging Retina. 2010 Jul 1;41(4):467-71.
- ↑ Jump up to: 4.0 4.1 4.2 4.3 Hankinson EV, Lyons CJ, Hukin J, Cochrane DD. Ophthalmological outcomes of patients treated for pineal region tumors. Journal of Neurosurgery: Pediatrics. 2016 May 1;17(5):558-63.
- ↑ Jump up to: 5.0 5.1 5.2 Hoehn ME, Calderwood J, O'Donnell T, Armstrong GT, Gajjar A. Children with dorsal midbrain syndrome as a result of pineal tumors. Journal of American Association for Pediatric Ophthalmology and Strabismus. 2017 Feb 1;21(1):34-8.
- ↑ Jump up to: 6.0 6.1 Daroff, R, Jankovic, J, Mazziotta, J, Pomeroy, S. Bradley's neurology in clinical practice. London: Elsevier; 2016; 206.
- ↑ Jump up to: 7.0 7.1 7.2 7.3 7.4 7.5 Keane JR. The pretectal syndrome: 206 patients. Neurology. 1990 Apr 1;40(4):684.
- ↑ Iorio-Morin C, Kano H, Huang M, Lunsford LD, Simonová G, Liscak R, Cohen-Inbar O, Sheehan J, Lee CC, Wu HM, Mathieu D. Histology-stratified tumor control and patient survival after stereotactic radiosurgery for pineal region tumors: A report from the international gamma knife research foundation. World neurosurgery. 2017 Nov 1;107:974-82.
- ↑ Jump up to: 9.0 9.1 9.2 9.3 9.4 9.5 Leigh RJ, Zee DS. The neurology of eye movements. New York: Oxford University Press; 2015; 877– 887.
- ↑ Jump up to: 10.0 10.1 10.2 10.3 10.4 10.5 Moss HE, Goodwin J. Vertical gaze palsy. [Updated 2019 Jan 10]. MedLink Neurology: The Information Resource for Clinical Neurology [Internet]. 2001 July 19. Available from: http://www.medlink.com/article/vertical_gaze_palsy
- ↑ Jump up to: 11.0 11.1 11.2 11.3 11.4 11.5 Feroze KB, Patel BC. Parinaud Syndrome. [Updated 2019 Jan 13]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2019 Jan. Available from: https://www.ncbi.nlm.nih.gov/books/NBK441892/
- ↑ Jump up to: 12.0 12.1 Convergence-retraction nystagmus [Internet]. Available from: https://www.aao.org/bcscsnippetdetail.aspx?id=ccf5cca4-5f91-4c25-9711-18ca76f8ced1
- ↑ Pollak L, Zehavi‐Dorin T, Eyal A, Milo R, Huna‐Baron R. Parinaud syndrome: Any clinicoradiological correlation?. Acta Neurologica Scandinavica. 2017 Dec;136(6):721-6.
- ↑ Swash M. Periaqueductal dysfunction (the Sylvian aqueduct syndrome): a sign of hydrocephalus?. Journal of Neurology, Neurosurgery & Psychiatry. 1974 Jan 1;37(1):21-6.
- ↑ Jump up to: 15.0 15.1 Buckley EG, Holgado S. Surgical treatment of upgaze palsy in Parinaud's syndrome. Journal of American Association for Pediatric Ophthalmology and Strabismus. 2004 Jun 1;8(3):249-53.