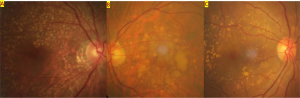
Pachydrusen
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
Disease Entity
Pachydrusen, a very recently described entity, are seen in the pachychoroid spectrum, which are diseases that share the finding of increased choroidal thickness. These deposits deviate from the typical presentation of soft drusen and pseudodrusen. As a result, the characterization of drusenoid deposits can offer insight into the development of subtypes in either AMD or pachychoroid disease. However, due to the high degree of clinical overlap present in such conditions, further research is necessary to strengthen the predictive use of differentiating these lesions.
Clinical Features
The term “pachydrusen” was introduced by Spaide (2018) to describe pachychoroid-associated drusen.[1] Such lesions differed in both appearance and distribution of soft drusen, a common finding in non-exudative AMD. Pachydrusen are large (typically >125 µm), sub-RPE deposits that are yellow-white in color. Additionally, the deposits are distributed across the posterior pole and are isolated or clustered in small groups. The deposits appear with irregular, complex shapes but have distinct borders. Another important distinguishing feature is that these drusenoid lesions are associated with the presence of thickened choroid.
In contrast, soft drusen are located underneath RPE and in the inner collagenous layer of Bruch's membrane.[2] These are typically ovoid, yellow-white deposits with poorly defined borders. Soft drusen are concentrated at the central macula and appear decreasingly towards the peripheral retina. Typically, soft drusen are seen in association with thin choroid, but normal choroid can also be present. Clinically, soft drusen is a characteristic of non exudative age-related macular degeneration (AMD). Pachydrusen, however, is related to the pachychoroid diseases such as central serous chorioretinopathy and polypoidal choroidal vasculopathy, are >125 μm solitary drusenoid deposits with thickened underlying choroid. Pseudodrusen also known as subretinal drusenoid deposits, are another distinguishable type of deposit. These are spotted, yellow-white deposits found above the RPE and can be associated with either thin or normal choroid. Diseases associated with these lesions include geographical atrophy and type 2 and 3 macular neovascularization, while pachydrusen is associated with type-1 macular neovascularization.
Risk Factors
The development of pachydrusen is not well understood.[2][3] [4] Certain risk factors, however, have been identified. For instance, ARMS2 A69S is a risk allele associated with AMD. However, the risk allele frequency was lower in groups of AMD patients with pachydrusen compared to groups with soft drusen and pseudodrusen. Additionally, CFH risk alleles for AMD have been shown to be protective for pachychoroid disease such as central serous chorioretinopathy.
Epidemiology
The prevalence of pachydrusen and associated factors have been noted in a variety of studies in cohorts of patients diagnosed with AMD or pachychoroid disease. Listed below are a summary of such studies organized by disease.
Age-Related Macular Degeneration
Spaide (2018) examined 94 eyes of 71 primarily Caucasian patients with non exudative AMD.[1] Only 11.7% of eyes had pachydrusen, while soft drusen and subretinal drusenoid deposits were found in 47.9% and 40.4% of eyes, respectively. The eyes with pachydrusen had a mean subfoveal choroidal thickness of 419 µm, significantly greater than the soft drusen group and the subretinal drusenoid deposit group. In the study by Singh et al. (2019), 8.4% of eyes with dry AMD in an Indian cohort contained pachydrusen, with a mean subfoveal choroidal thickness of 289.66 µm. [5]
Polypoidal Choroidal Vascularization
Lee & Suk (2019) examined the prevalence of pachydrusen in patients with polypoidal choroidal vascularization.[6] 169 eyes from 90 patients were examined from a South Korean cohort. Pachydrusen was found in 49.3% of eyes, while soft drusen and subretinal drusenoid deposits were found in 12.3% and 6.9% of eyes, respectively. Mean subfoveal choroidal thickness of pachydrusen eyes was 403.1 µm. Additionally, 41.7% of eyes with pachydrusen demonstrated choroidal vascular hyperpermeability, while no eyes in the soft drusen or subretinal drusenoid deposit groups had this finding. Pachyvessel morphology was also observed more frequently in the pachydrusen group, which was observed in 80.6% of these eyes. Sheth et al. (2020) examined 131 eyes from 79 patients with a pachychoroid disease. 50 eyes had polypoidal choroidal vascularization, of which 56% had pachydrusen. Kang et al. (2021) examined 87 eyes with polypoidal choroidal vascularization. Pachydrusen was noted in 49.4% of eyes.[7]
Pachychoroid Neovasculopathy
Of the 18 eyes studied by Kang et al. (2021) with pachychoroid neovasculopathy, 61.1% had pachydrusen. Notably, 72.2% had choroidal vascular hyperpermeability and 72.2% had punctate hyperfluorescent spots.[7]
Central Serous Chorioretinopathy
Takahashi et al. (2020) compared 614 eyes with central serous chorioretinopathy to 1640 control eyes in a Japanese population.[8] Pachydrusen was prevalent in 40.1% of central serous chorioretinopathy eyes, compared to 15.6% control eyes. Sheth et al. (2020) studied 45 eyes with central serous chorioretinopathy, of which 60% had pachydrusen.[9]
Pachychoroid Pigment Epitheliopathy
In the study by Sheth et al. (2020), pachydrusen was observed in 41.67% of 36 eyes with pachychoroid pigment epitheliopathy.
Diagnosis
Physical examination
On fundoscopic exam, pachydrusen can be seen and differentiated from other drusenoid lesions by examining the appearance, distribution, and clustering of deposits. Since this finding is found in pachychoroid disease, other findings such as reduced choroidal vascular markings can be found as well pigment epithelium detachments.[2][10]
Additional Testing
- Optical Coherence tomography (OCT) to observe pachydrusen changes and choroidal morphology.[10] Swept Source OCT can be used for better resolution of microvasculature in assessment of pachychoroid diseases.
- Fundus Fluorescein Angiography (FFA) and Indocyanine Green Angiography (ICGA) to assess for hyperfluorescent changes, which have been associated with pachydrusen. [2]
Differential diagnosis
- Pachychoroid disease spectrum,
- Pachychoroid neovasculopathy,
- Pachychoroid pigment epitheliopathy,
- Central serous chorioretinopathy,
- Polypoidal choroidal vasculopathy,
- Focal Choroidal Excavation,
- Peripapillary Pachychoroid Syndrome
- Age-related macular degeneration
Management
Pachydrusen were first described in 2018; hence, data regarding the natural history of this entity is limited. Further studies are needed to understand their clinical significance and their relationship with exudative changes. Sheth et al. (2020) observed the progression of pachydrusen to polypoidal choroidal vasculopathy with choroidal neovascular membranes in 32.14% of eyes with pachychoroid disease. Additionally, the authors noted progression of disease activity, as increased leakage was noted from areas with pachydrusen. Such developments suggest that pachydrusen is a risk factor for visual morbidity. [9]
References
- ↑ 1.0 1.1 Spaide, R. F. (2018). Disease expression in nonexudative age-related macular degeneration varies with choroidal thickness. Retina, 38(4), 708-716.
- ↑ 2.0 2.1 2.2 2.3 Zhang, X., & Sivaprasad, S. (2020). Drusen and pachydrusen: the definition, pathogenesis, and clinical significance. Eye, 1-13.
- ↑ Fukuda, Y., Sakurada, Y., Yoneyama, S., Kikushima, W., Sugiyama, A., Matsubara, M., ... & Iijima, H. (2019). Clinical and genetic characteristics of pachydrusen in patients with exudative age-related macular degeneration. Scientific reports, 9(1), 1-7.
- ↑ Hosoda, Y., Yoshikawa, M., Miyake, M., Tabara, Y., Ahn, J., Woo, S. J., ... & Nagahama Study Group. (2018). CFH and VIPR2 as susceptibility loci in choroidal thickness and pachychoroid disease central serous chorioretinopathy. Proceedings of the National Academy of Sciences, 115(24), 6261-6266.
- ↑ Singh, S. R., Oli, A., Mohan, S., Goud, A., Rasheed, M. A., Vupparaboina, K. K., & Chhablani, J. K. (2019). Pachydrusen in Indian population: A hospital-based study. Indian journal of ophthalmology, 67(3), 371.
- ↑ Lee, J., & Byeon, S. H. (2019). Prevalence and clinical characteristics of pachydrusen in polypoidal choroidal vasculopathy: multimodal image study. Retina, 39(4), 670-678.
- ↑ 7.0 7.1 Kang, H. G., Han, J. Y., Kim, M., Byeon, S. H., Kim, S. S., Koh, H. J., & Lee, C. S. (2021). Pachydrusen, choroidal vascular hyperpermeability, and punctate hyperfluorescent spots. Graefe's Archive for Clinical and Experimental Ophthalmology, 1-10.
- ↑ Takahashi, A., Hosoda, Y., Miyake, M., Miyata, M., Oishi, A., Tamura, H., ... & Tsujikawa, A. (2020). Clinical and Genetic Characteristics of Pachydrusen in Eyes with Central Serous Chorioretinopathy and General Japanese Individuals. Ophthalmology Retina.
- ↑ 9.0 9.1 Sheth, J., Anantharaman, G., Kumar, N., Parachuri, N., Bandello, F., Kuppermann, B. D., ... & Sharma, A. (2020). Pachydrusen: the epidemiology of pachydrusen and its relevance to progression of pachychoroid disease spectrum. Eye, 34(9), 1501-1503.
- ↑ 10.0 10.1 Baek, J., Lee, J. H., Chung, B. J., Lee, K., & Lee, W. K. (2019). Choroidal morphology under pachydrusen. Clinical & experimental ophthalmology, 47(4), 498-504.