Oxidative Stress in Ophthalmology
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
Disease entity
Oxidative stress in ocular diseases
What is oxidative stress
It is an imbalance between the generation of free radicals and the ability of antioxidants to neutralize them. Normally, cells produce metabolic-derived end products and generate energy by reducing molecular oxygen to water. During this process, small amounts of partially reduced reactive oxygen species are produced as byproducts of mitochondrial respiration. Reactive oxygen species (ROS) can damage the component of the cell (lipid, proteins, and nucleic acid). Cells have an antioxidant system to counter the effect of ROS.[1]
Cellular metabolism
In ocular tissue, metabolism occurs in the cornea, natural crystalline lens, retinal pigment epithelium. Glucose is metabolized through series of cellular cycles (glycolysis, Krebs cycle, hexose monophosphate shunt, sorbitol pathway). ATP and NADPH are byproducts of cellular metabolic activities and maintain cellular homeostasis.[2]
Oxidants and antioxidants mechanism in eye
Free radicals like superoxide anion, hydroxyl ion, hydroperoxyl, lipid peroxyl, singlet oxygen, hydrogen peroxide are generated from different sources. The endogenous sources of ROS include cellular metabolic activities, inflammation, respiratory burst (NADPH oxidase). The exogenous sources of ROS include ultraviolet, ionizing, gamma radiation, and x rays. These radiations are capable to interact with ocular tissue and generate reactive oxygen species. Nitrogen dioxide and nitrates are generated by macrophage.[3]
Antioxidant system includes[1][4]
- Vitamin A, C, E, beta-carotene
- Enzyme system:
- a) Mitochondria: Superoxide dismutase, glutathione peroxidase
- b) Peroxisome: Catalase
- c) Cytosol: Superoxide dismutase, glutathione peroxidase, ferritin, ceruloplasmin
- Minerals: Selenium, copper, zinc
Effects of ROS at cellular level[1]
- Lipid peroxidation within the plasma membrane
- DNA fragmentation
- Protein cross-linking and fragmentation
- Apoptosis of cell, necrosis, and autophagy response
Importance of the role of oxidative stress in ocular diseases
- Anterior segment disease: Dry eye disease, Meibomian gland disease, cataract
- Glaucoma
- Posterior segment disease: Age-related macular degeneration, diabetic retinopathy, retinitis pigmentosa, retinopathy of prematurity
- Other conditions: fungal keratitis at the experimental level
Dry eye disease and Meibomian gland disease
Aging, cigarette smoke, low humidity, sunlight, pollutants are risk factors to produce ROS. Loss of homeostasis, hyperosmolarity, ocular surface inflammation in dry eye disease releases pro-inflammatory cytokines (IL1, 6). Excess production of ROS can cause ocular surface damage [5].Normal tear film contain antioxidants like lactoferrin, ascorbate which protect the ocular surface against ROS.[6] The accumulation of ROS in ocular surface due superoxide dismutase deficiency lead to dry eye disease and meibomian gland disease. Studies show the role of oxidative damage in the dry eye disease spectrum (Sjogren's and non-Sjogren's syndrome):
| Author | Year | Conclusion |
|---|---|---|
| Cejkova. J[7] et al | 2008 | Decrease the expression of superoxide dismutase, catalase, and glutathione peroxidise in conjunctival epithelium occurs in Sjogrens syndrome associated dry eye disease. |
| Wakamatsu[8] et al | 2013 | Oxidative stress marker (lipid peroxidation) has role in pathogenesis of Sjogrens syndrome associated dry eye disease. |
| Choi[9] et al | 2016 | Increase in level of malondialdehyde in tear film occurs in non Sjogrens syndrome associated dry eye disease. |
Glaucoma
Raised intraocular pressure inhibits axoplasmic flow across the optic nerve. An excess amount of ROS production can cause retinal ganglion cell apoptosis and trabecular meshwork damage. Oxidative damage of trabecular meshwork can alter aqueous outflow.[10] Antioxidative agents are the future mode of treatment in the prevention of glaucoma progression.
Cataract
A natural crystalline lens is a transparent avascular structure. Different metabolic activities, antioxidative mechanism, and Na+ K+ pump helps in keeping the lens clear. Radiation, aging, diabetes causes reduction of antioxidant enzymes leads to cataract.[2]
Role of free radical formation and its neutralization in the lens
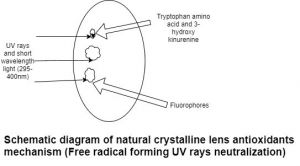
Lens receives O2 from the aqueous humor. Glucose metabolized in the lens through different pathways like anaerobic glycolysis, Krebs cycle, hexose monophosphate shunt, sorbitol pathway. These cellular activities produce reactive oxygen species. Sunlight absorbed by photosensitizer undergoes photoexcitation (photoexcited singlet state converted into intermediate excited triplet state). This triplet state allow for interaction with another molecule to produce ROS. Antioxidants such as glutathione peroxidase, catalase, superoxide dismutase, and ascorbate present in lens epithelium and fibers. [2]Glutathione peroxidase neutralizes hydrogen peroxide and lipid peroxide. Ascorbate present in outer layers of lens reacts with superoxide, peroxide, hydroxyl ion and produces dehydroascorbate product. Ascorbate also scavenges singlet oxygen and thiol radical.[11] Aromatic amino acid (Tryptophan), fluorophores and 3-hydroxykinurenine provide protection against UV rays in the lens. (Fig.1)
Action of reactive oxygen species in lens[11]
- Polymerization and cross-linkage of crystallin proteins. Finally, lead to inactivation of catalase and glutathione enzyme.
- Membrane lipid peroxidation leads to the formation of malondialdehyde.
- DNA base modification (8 hydroxy guanosine) and distortion of DNA helical structure at 8,5 cyclopurine deoxyribonucleosides.
Retinal disorders
RPE-Bruchs complex act as a barrier and plays a key role in the management of metabolic waste and oxidative stress. Studies show dysregulation of the antioxidant enzyme system leads to retinal conditions like age-related macular degeneration, diabetic retinopathy, retinitis pigmentosa, retina of prematurity.
Diabetic retinopathy
Diabetic retinopathy is a microvascular complication of diabetes. Abnormal glucose metabolism produces advanced glycation end products which further get accumulate in the retinal vascular cell. Increase signaling to caspase 3 causes retinal capillary cell apoptosis.[1] [12]Decrease of the level of the antioxidant enzyme in retinal tissue produces ROS which further release pro-inflammatory cytokines lead to dysfunction of the vascular endothelial cell. Vascular permeability and increase in expression of VEGF cause retinal neovascularization. Excess glucose convert into sorbitol by aldose reductase which needs NADPH. Further sorbitol transforms into fructose by sorbitol dehydrogenase. Increase the metabolism through the polyol pathway cause consumption of NADPH( Nicotinamide adenine dinucleotide) and decrease of its availability for regeneration of glutathione enzyme system.[2]
Age-related macular degeneration (ARMD) is a progressive, degenerative disorder principally affects retinal pigment epithelium, Bruch's membrane and choriocapillaris. Its pathogenesis involves oxidative damage to the RPE-Bruchs complex. Constant exposure to ultraviolet rays leads to the production of ROS and oxidative damage to RPE-Bruch's complex. The thickening of the collagenous layer within Bruch's membrane and calcification (elastin, collagen degeneration) occurs after the damage of RPE-Bruch’s complex[13]. An increase in advanced glycation end products and a decrease in antioxidant mechanism make the RPE-Bruchs barrier more hydrophobic which further, impedes nutrients and fluid passage through the outer retina and choroid.
Retinitis Pigmentosa
It is a hereditary disease that is characterized by degeneration of photoreceptors (rod-cone dystrophy). Studies have been carried out to find out the role of oxidative stress in retinal pigment epithelium degeneration.[14] The metabolic support of photoreceptors hampered, due to a defective antioxidant mechanism. Recent studies explained the role of autophagy and oxidative stress in retinitis pigmentosa. Shen et al carried out a study in transgenic pigs and explained the cause of cone degeneration in RP. They found cone degeneration is because of oxidative damage produced by hyperoxia in the outer retina due to decreased oxygen consumption in rod receptors death.[15]
Retinopathy of Prematurity (ROP)
Niesman et al explained the role of antioxidants in ROP in an animal model. In humans role of it in ROP is still in the investigation phase.[16]
Other conditions
Fungal Keratitis
Role of oxidative damage in the pathogenesis of fungal keratitis is at experimental stage.[17]
References
- ↑ 1.0 1.1 1.2 1.3 Kumar V, Abbas AK, Fausto N, Aster JC. Robbins and Cotran pathologic basis of disease.10th ed.Philadelphia:Elsevier health sciences (p)Ltd; 2021.P.52-54.
- ↑ 2.0 2.1 2.2 2.3 Khurana A.K .Disorders of lens and cataract surgery.1st ed. New Delhi: CBS publishers (p) Ltd; 2020.P.59-65.
- ↑ Su LJ, Zhang JH, Gomez H, Murugan R, Hong X, Xu D, Jiang F, Peng ZY. Reactive oxygen species-induced lipid peroxidation in apoptosis, autophagy, and ferroptosis. Oxidative medicine and cellular longevity. 2019 Oct 13;2019.
- ↑ Ganea E, Harding JJ. Glutathione-related enzymes and the eye. Current eye research. 2006 Jan 1;31(1):1-1.
- ↑ Seen S, Tong L. Dry eye disease and oxidative stress. Acta ophthalmologica. 2018 Jun;96(4):e412-20.
- ↑ Ohashi Y, Dogru M, Tsubota K. Laboratory findings in tear fluid analysis. Clinica chimica acta. 2006 Jul 15;369(1):17-28.
- ↑ Cejkova J, Ardan T, Simonova Z, Cejka C, Malec J, Dotrelova D, Brunová B. Decreased expression of antioxidant enzymes in the conjunctival epithelium of dry eye (Sjogren s syndrome) and its possible contribution to the development of ocular surface oxidative injuries. Histology and histopathology. 2008.
- ↑ Wakamatsu TH, Dogru M, Matsumoto Y, Kojima T, Kaido M, Ibrahim OM, Sato EA, Igarashi A, Ichihashi Y, Satake Y, Shimazaki J. Evaluation of lipid oxidative stress status in Sjögren syndrome patients. Investigative ophthalmology & visual science. 2013 Jan 1;54(1):201-10.
- ↑ Choi W, Lian C, Ying L, Kim GE, You IC, Park SH, Yoon KC. Expression of lipid peroxidation markers in the tear film and ocular surface of patients with non-Sjogren syndrome: potential biomarkers for dry eye disease. Current eye research. 2016 Sep 1;41(9):1143-9.
- ↑ Pulliero A, Seydel A, Camoirano A, Saccà SC, Sandri M, Izzotti A. Oxidative damage and autophagy in the human trabecular meshwork as related with ageing. PloS one. 2014 Jun 19;9(6):e98106.
- ↑ 11.0 11.1 Yanoff M, Duckers J. Ophthalmology.4th ed.China: Elsevier Saunders publishers (p) Ltd; 2014. p.330.e1-330e11.
- ↑ Madsen-Bouterse SA, Kowluru RA. Oxidative stress and diabetic retinopathy: pathophysiological mechanisms and treatment perspectives. Reviews in Endocrine and Metabolic Disorders. 2008 Dec;9(4):315-27.
- ↑ Bellezza I. Oxidative stress in age-related macular degeneration: Nrf2 as therapeutic target. Frontiers in pharmacology. 2018 Nov 5;9:1280.
- ↑ Moreno ML, Mérida S, Bosch-Morell F, Miranda M, Villar VM. Autophagy dysfunction and oxidative stress, two related mechanisms implicated in retinitis pigmentosa. Frontiers in physiology. 2018 Jul 26;9:1008.
- ↑ Shen J, Yang X, Dong A, Petters RM, Peng YW, Wong F, Campochiaro PA. Oxidative damage is a potential cause of cone cell death in retinitis pigmentosa. Journal of cellular physiology. 2005 Jun;203(3):457-64.
- ↑ Niesman MR, Johnson KA, Penn JS. Therapeutic effect of liposomal superoxide dismutase in an animal model of retinopathy of prematurity. Neurochemical research. 1997 May;22(5):597-605.
- ↑ Hua X, Chi W, Su L, Li J, Zhang Z, Yuan X. ROS-induced oxidative injury involved in pathogenesis of fungal keratitis via p38 MAPK activation. Scientific reports. 2017 Sep 5;7(1):1-2.