Outflow Pathway Imaging
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
Introduction
Glaucoma is the major cause of irreversible vision loss worldwide.[1] Elevated intraocular pressure (IOP) represents the most important risk factor for glaucoma. IOP is regulated through aqueous drainage from the trabecular meshwork (TM) through the Schlemm’s canal (SC) draining into the collector channels (CCs), aqueous veins, and episcleral venous plexus (conventional outflow pathway).[2] Aqueous drainage occurs to a lesser extent through the uveoscleral pathway. The juxtacanalicular tissue of the TM and the SC represent the major sites of aqueous outflow resistance.[3] Decreased aqueous drainage results in elevated IOP which, if left untreated, may leads to glaucoma. Recently, with the emerging emergence of minimally (or micro-) invasive glaucoma surgeries (MIGS), a lot of interest has been developed for aqueous outflow imaging. Advances in aqueous outflow imaging may allow for identification of optimal treatment targets and also aid in procedure selection on a personalized level. In this article, we will review the recent advances in imaging the different structures of the conventional aqueous drainage system.
Anterior Chamber Angle
Gonioscopy is the gold standard in the evaluation of the anterior chamber angle (ACA) structures and differentiating between open vs. angle-closure glaucoma. In the last years, several imaging modalities have been developed to evaluate the ACA including ultrasound biomicroscopy (UBM), anterior segment optical coherence tomography (AS-OCT), and gonio-photography (GP). These methods allow the assessment of the ACA structures in a more quantitative and objective manner.[4]
Ultrasound biomicroscopy
In 1989, Pavlin and colleagues developed high-frequency UBM as a non-invasive method for evaluating anterior segment structures. [5] The UBM uses frequencies up to 50-100 MHz which allow for high image resolution however, the penetration is poor. Some factors such as room illumination, operator experience, and failure to control accommodation may affect the quality of images acquired by UBM. Barkana and colleagues evaluated iridotrabecular meshwork contact (ITC) in light and dark conditions using UBM. They reported that 17 out of 18 eyes demonstrated ITC in at least 1 angle quadrant at darkroom UBM, however, only 10 eyes had ITC under normal room illumination.[6] Several parameters have been proposed for the evaluation of ACA using UBM. These parameters include angle opening distance (AOD), trabecular-iris angle (TIA), and trabecular-ciliary process distance (TCPD) (Figure 1).[7] AOD is defined as the distance between the TM and the iris at 500 or 750 um anterior to the scleral spur (SS). TIA is defined as the angle of the angle recess, while TCPD is defined as the distance between the TM and the ciliary process at 500 um anterior to the SS.
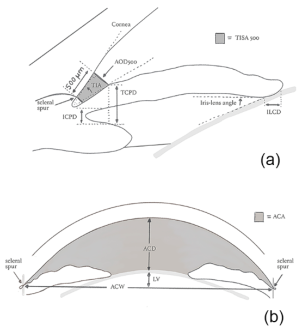
In a cohort of Chinese primary angle-closure suspect (PACS) patients, Kong and colleagues evaluated the agreement level between the visibility of the TM using gonioscopy and the ITC using UBM. They reported that more ITC was detected on UBM compared to gonioscopy.[8] Radhakrishnan and colleagues compared AS-OCT to UBM for the detection of narrow ACA. They demonstrate that both devices had similar mean values, reproducibility, sensitivity, and specificity in evaluating ACA parameters.[9] Another advantage of UBM in glaucoma is the evaluation of iris concavity in pigment dispersion syndrome, plateau iris configuration (Figure 2), and ciliary body lesions such as tumors or cysts which may cause secondary glaucoma.[7]
Anterior segment optical coherence tomography
AS-OCT is a non-invasive method that uses low-coherence interferometry to provide high-resolution images of the anterior segment structures allowing for quantitative analysis. It can be time-domain (TD-OCT), spectral-domain (SD-OCT), or more recently swept-source (SS-OCT). Compared to UBM, it has the advantages of faster acquisition and higher quality of the images.
Several studies have demonstrated a higher tendency of AS-OCT to detect angle closure compared to slit-lamp gonioscopy. This could be explained by the ability of the AS-OCT to evaluate ACA using infrared rays without causing indentation which may inadvertently open the ACA during gonioscopic evaluation. Another factor is that small ITC may not be graded as angle-closure during gonioscopic evaluation.[10][11]
In a study by Nolan et al. on 342 eyes comparing the agreement level between AS-OCT and gonioscopy in the diagnosis of angle-closure, they demonstrated that 66.7% and 44.4% had angle closure with AS-OCT and gonioscopy, respectively.[10] Another study reported a statistically significant discrepancy in the diagnosis of angle-closure (defined as invisible posterior pigmented TM on gonioscopy in ≥1 quadrant) between AS-OCT and gonioscopy. The angle-closure was detected in 59% using AS-OCT compared to 33% using gonioscopy. [11]
Baskaran and colleagues evaluated 342 patients with open-angles at gonioscopy but with ITC at AS-OCT and followed them up for 4 years. They reported that the more quadrants of ITC on AS-OCT, the higher the chance to develop angle-closure.[12]
Several parameters have been proposed for the evaluation of ACA using AS-OCT. These parameters include AOD and trabecular iris space area (TISA). TISA is the trapezoid area bounded by AOD, the anterior iris surface, and the inner corneoscleral wall. The best landmark for ACA measurements is SS, however, it has been demonstrated that it may not be identified in 15-28% of the AS-OCT, especially in narrow angles.[13] The interobserver variability in detecting SS accounts for variability in the TISA measurements. It has been reported that for every 0.1 mm decrease in AOD705, there were increased odds of angle-closure by 3.27.[14]
SS-OCT provides higher resolution images which could make the identification of SS easier with less TISA variability. SS-OCT allows for 360 degrees evaluation of ACA and the extent of angle closure. Baskaran and colleagues evaluated the extent of ITC across the ACA evaluating the extent of angle-closure (ITC index) using built-in software. They demonstrated that median ITC indices were 15.2% and 48.5% for gonioscopically open-angle and closed-angles, respectively.[15] Gold and colleagues used SS-OCT to evaluate the aging effects on TM. They evaluated several measurements including TM area, TM length, and TM interface shadow length and area. They reported no correlation between TM measurements and age; however, the area of TM interface shadow was increased with age.[16] SS-OCT has been utilized to measure the area and the extent of peripheral anterior synechiae (PAS). In a cross-sectional study conducted by Lai and colleagues, there was a good agreement level (k=0.79) of PAS measurements between gonioscopy and SS-OCT.[17] Tun and colleagues demonstrated a reduction of ITC in patients with primary angle-closure glaucoma (PACG) who underwent cataract extraction and goniosynechiolysis. peripheral anterior synechiae.[18]
Gonio-photographic system
EyeCam
EyeCam (Clarity Medical Systems, Pleasanton CA, USA) is a contact portable hand-held device for ACA evaluation. The patient has to lie supine during the whole period of examination and is asked to look opposite to the probe position. Several studies showed that EyeCam can achieve clear ACA images in ≥ 98% of patients with a good agreement between EyeCam and gonioscopy.[19] Perera and colleagues reported agreement levels between the gonioscopy and EyeCam of 0.73 for the superior angle, 0.75 for the inferior angle, 0.76 for the nasal angle, and 0.72 for the temporal angle. However, more eyes were diagnosed with closed-angle (defined as invisible TM in ≥1 quadrant) with the EyeCam (27% of eyes) compared to gonioscopy (13.8%o of eyes) and the difference was statistically significant.[20] Another study compared between EyeCam and AS-OCT and showed a statistically significant higher sensitivity and specificity of EyeCam compared to AS-OCT In the assessment of angle-closure (defined as ≥2 closed angles on gonioscopy).[19] A significant disadvantage of the EyeCam is the inability to perform indentation.
NGS-1 automated gonioscope
NSG-1 gonioscope is a novel method that provides gonioscopic images of the entire 360 degrees of the ACA. It has a high-resolution camera that takes multiple pictures at various focal depths. Using NSG-1 gonioscope needs patient cooperation and eye fixation. A major disadvantage is that images may be of poor quality. A study by Teixeira and colleagues reported low-quality images in 22.5% of cases and low sensitivity compared to gonioscopy.[21]
Deep Learning
Recently, few deep learning systems have been developed for automated detection of angle-closure using AS-OCT images.[22] [23] Fu and colleagues reported an area under a curve of 0.96 with 0.9 sensitivity and 0.92 specificity for their deep learning model.[23][24]
Schlemm’s Canal
Ultrasound biomicroscopy
A study by Yan and colleagues studied the SC and TM in patients with primary open-angle glaucoma (POAG) compared to a normal population using high-frequency UBM. They demonstrated that POAG eyes had smaller SC meridional diameter, SC coronal diameter, and TM thickness (p<0.001) compared to normal eyes. They compared POAG eyes with elevated IOP to those with normal IOP and reported that the former group had a statistically significant smaller SC coronal diameter and TM thickness but not SC meridional diameter.[25] Another study was conducted on glaucomatous and non-glaucomatous children aged <18 years. They reported that the mean SC diameter was 142 um in non-glaucomatous eyes compared to 64.9 in glaucomatous eyes (p = 0.007). They demonstrated that SC diameter varied by age being smaller in children aged <2 years.[26]
Anterior segment optical coherence tomography
Recent advances in OCT technology allowed for high-quality imaging of the SC. The quantitative metrics such as SC cross-sectional area (CSA) may differ from one OCT device to the other. A recent prospective study by Wu and colleagues included 10 normal patients with a mean age of 33 years and found that the CSA measured by Cirrus SD-OCT was larger than that measured by PLEX Elite SS-OCT or CASIA SS-OCT. In healthy Caucasian children, SC could be identified using SD-OCT and its metrics were not different according to the gender or spherical refractive error. They did not find a significant correlation between SC area and ACA or TM parameters. However, SC was identified in 73.8% of patients >15 years of age compared to 53.6% of patients ≤ 7 years of age.[27] Chen and colleagues enrolled 114 healthy subjects ranging from 7 to 83 years to investigate the morphological changes in SC and TM with aging. They demonstrated a statistically significant reduced SC size and detection rates with aging. TM thickness was positively correlated with age.[28]
Hong and colleagues prospectively compared the SC features in POAG to those of normal eyes using SD-OCT. They reported that POAG eyes had a significantly smaller SC area (11332 um) compared to normal eyes (13991 um) but there was no significant difference in the SC diameter (p=0.195). They investigated the correlation between the mean IOP and SC parameters and reported a significant correlation between the mean IOP and SC area but not SC diameter. There was no significant correlation between SC metrics and mean deviation in the visual field.[29] Several studies have investigated the morphological changes in the SC in response to topical medications, laser trabeculoplasty, and different types of glaucoma surgery using SD-OCT.
Topical medications
Chen and colleagues have demonstrated increased mean SC area by >90% in normal individuals eight hours after instillation of travoprost 0.004%. Increased SC area was maintained up to 84 hours after travoprost administration.[30] Another study investigated the effect of topical pilocarpine 1% and 2% and showed expansion of SC in response to pilocarpine in both healthy and glaucomatous eyes.[31] On the other hand, Park and colleagues reported no changes in the TM width, TM thickness, and SC area in POAG eyes 3 months after receiving a combination of timolol/dorzolamide. However, in POAG eyes who received prostaglandin analogs, there was a significant increase in the TM thickness and SC area.[32] In healthy eyes, Rosman and colleagues reported decreased SC CSA after topical cyclopentolate 1% with more reduction in eyes with larger baseline CSA, however, there was no reduction in the mean IOP.[33]
Laser trabeculoplasty
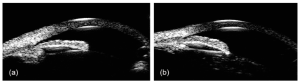
Using enhanced depth imaging OCT, Skaat and colleagues studied the changes in SC after selective laser trabeculoplasty (SLT) in 13 POAG eyes. They demonstrated a statistically significant increase in the mean SC CSA and the mean SC volume after SLT. There was a significant positive correlation between increased SC CSA and IOP reduction after SLT.[34] Another study by Varshney and colleagues showed that SC was identified in 90% of juvenile open-angle glaucoma (JOAG) eyes who underwent SLT and had successful IOP reduction. However, SC was identified in only 26.6% of patients who failed to respond to SLT (Figure 2). The authors concluded that identification of SC was a strong predictor of successful IOP reduction after SLT in JOAG eyes.[35]
Glaucoma surgery
Kuerten and colleagues analyzed the long-term anatomic changes in the anterior segment in 15 patients who underwent canaloplasty using AS-OCT and UBM. The mean follow-up was 20±4.9 after canaloplasty. They demonstrated a postoperative increase in SC height (+351%) and width (+144%).[36]
In a study including 40 PACG patients who underwent trabeculectomy, Hong and colleagues showed a significant postoperative increase in the SC diameter and area compared to preoperative values. The changes in SC were correlated with the percentage of IOP reduction.[37]
Collector Channels
Few imaging studies have evaluated the CCs in-vivo.[38] A study by Li and colleagues evaluated the CCs connected SC using enhanced depth imaging OCT. They included 11 patients with a mean age of 28±5 years. They demonstrated variable numbers of CCs in the studied area ranging from 5 to 11 with a significantly larger number of CCs nasally (5.5±1.4) compared to temporally (3.3±1.1) (p=0.001). The SC CSA was significantly larger in areas with more CCs (r=0.6).[39]
Intrascleral and Conjunctival Vasculature
Meyer and Watson suggested reducing the dose of injected fluorescein to be able to visualize episcleral and conjunctival vasculature by reducing leakage.[40] In a recent prospective study including 10 healthy subjects, Akagi and colleagues used AS-OCT angiography for the evaluation of conjunctival vessels and deeper intrascleral vessels. They have evaluated the vessel density, length density, diameter index, and fractal dimension in the nasal, temporal, superior, and inferior quadrants. All the 4 parameters were different in the 4 quadrants in the deep vascular layer, however, in the superficial layer only the vessel diameter index was significantly different between the 4 quadrants being highest in the nasal quadrant (p=0.003).[41]
Conclusions
Over the last few years, aqueous outflow imaging has improved which may allow for identification of optimal surgical treatment target for glaucoma patients. It may also help to identify patients at high risk to develop angle-closure disease. Advances in deep learning algorithms in the next years may allow easier screening this population of patients.
References
- ↑ Quigley HA, Broman AT. The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol. 2006;90(3):262-7.
- ↑ Grant WM. Clinical measurements of aqueous outflow. Am J Ophthalmol. 1951;34(11):1603-5.
- ↑ Mäepea O, Bill A. Pressures in the juxtacanalicular tissue and Schlemm's canal in monkeys. Exp Eye Res. 1992;54(6):879-83.
- ↑ Porporato N, Baskaran M, Husain R, Aung T. Recent advances in anterior chamber angle imaging. Eye (Lond). 2020;34(1):51-9
- ↑ Pavlin CJ, Harasiewicz K, Sherar MD, Foster FS. Clinical use of ultrasound biomicroscopy. Ophthalmology. 1991;98(3):287-95
- ↑ Barkana Y, Dorairaj SK, Gerber Y, Liebmann JM, Ritch R. Agreement between gonioscopy and ultrasound biomicroscopy in detecting iridotrabecular apposition. Arch Ophthalmol. 2007;125(10):1331-5
- ↑ Jump up to: 7.0 7.1 Dada T, Gadia R, Sharma A, Ichhpujani P, Bali SJ, Bhartiya S, et al. Ultrasound biomicroscopy in glaucoma. Surv Ophthalmol. 2011;56(5):433-50
- ↑ Kong X, Foster PJ, Huang Q, Zheng Y, Huang W, Cai X, et al. Appositional closure identified by ultrasound biomicroscopy in population-based primary angle-closure glaucoma suspects: the Liwan eye study. Invest Ophthalmol Vis Sci. 2011;52(7):3970-5
- ↑ Radhakrishnan S, Goldsmith J, Huang D, Westphal V, Dueker DK, Rollins AM, et al. Comparison of optical coherence tomography and ultrasound biomicroscopy for detection of narrow anterior chamber angles. Arch Ophthalmol. 2005;123(8):1053-9
- ↑ Jump up to: 10.0 10.1 Nolan WP, See JL, Chew PT, Friedman DS, Smith SD, Radhakrishnan S, et al. Detection of primary angle closure using anterior segment optical coherence tomography in Asian eyes. Ophthalmology. 2007;114(1):33-9
- ↑ Jump up to: 11.0 11.1 Sakata LM, Lavanya R, Friedman DS, Aung HT, Gao H, Kumar RS, et al. Comparison of gonioscopy and anterior segment ocular coherence tomography in detecting angle closure in different quadrants of the anterior chamber angle. Ophthalmology. 2008;115(5):769-74
- ↑ Baskaran M, Iyer JV, Narayanaswamy AK, He Y, Sakata LM, Wu R, et al. Anterior Segment Imaging Predicts Incident Gonioscopic Angle Closure. Ophthalmology. 2015;122(12):2380-4
- ↑ Wang BS, Narayanaswamy A, Amerasinghe N, Zheng C, He M, Chan YH, et al. Increased iris thickness and association with primary angle closure glaucoma. Br J Ophthalmol. 2011;95(1):46-50
- ↑ Nongpiur ME, Aboobakar IF, Baskaran M, Narayanaswamy A, Sakata LM, Wu R, et al. Association of Baseline Anterior Segment Parameters With the Development of Incident Gonioscopic Angle Closure. JAMA Ophthalmol. 2017;135(3):252-8
- ↑ Baskaran M, Ho SW, Tun TA, How AC, Perera SA, Friedman DS, et al. Assessment of circumferential angle-closure by the iris-trabecular contact index with swept-source optical coherence tomography. Ophthalmology. 2013;120(11):2226-31.
- ↑ Gold ME, Kansara S, Nagi KS, Bell NP, Blieden LS, Chuang AZ, et al. Age-related changes in trabecular meshwork imaging. Biomed Res Int. 2013;2013:295204
- ↑ Yang Y, Wu Y, Guo C, Han Y, Deng M, Lin H, et al. Diagnostic Performance of Deep Learning Classifiers in Measuring Peripheral Anterior Synechia Based on Swept Source Optical Coherence Tomography Images. Front Med (Lausanne). 2021;8:775711
- ↑ Tun TA, Baskaran M, Perera SA, Htoon HM, Aung T, Husain R. Swept-source optical coherence tomography assessment of iris-trabecular contact after phacoemulsification with or without goniosynechialysis in eyes with primary angle closure glaucoma. Br J Ophthalmol. 2015;99(7):927-31
- ↑ Jump up to: 19.0 19.1 Comparison of EyeCam and anterior segment optical coherence tomography in detecting angle closure. Acta Ophthalmol. 2012
- ↑ Perera SA, Baskaran M, Friedman DS, Tun TA, Htoon HM, Kumar RS, et al. Use of EyeCam for Imaging the Anterior Chamber Angle. Investigative Ophthalmology & Visual Science. 2010;51(6):2993-7
- ↑ Teixeira F, Sousa DC, Leal I, Barata A, Neves CM, Pinto LA. Automated gonioscopy photography for iridocorneal angle grading. Eur J Ophthalmol. 2020;30(1):112-8
- ↑ Xu BY, Chiang M, Chaudhary S, Kulkarni S, Pardeshi AA, Varma R. Deep Learning Classifiers for Automated Detection of Gonioscopic Angle Closure Based on Anterior Segment OCT Images. Am J Ophthalmol. 2019;208:273-80
- ↑ Jump up to: 23.0 23.1 Fu H, Baskaran M, Xu Y, Lin S, Wong DWK, Liu J, et al. A Deep Learning System for Automated Angle-Closure Detection in Anterior Segment Optical Coherence Tomography Images. Am J Ophthalmol. 2019;203:37-45
- ↑ Li F, Yang Y, Sun X, Qiu Z, Zhang S, Tun TA, et al. Digital Gonioscopy Based on Three-dimensional Anterior-Segment OCT: An International Multicenter Study. Ophthalmology. 2022;129(1):45-53
- ↑ Yan X, Li M, Chen Z, Zhu Y, Song Y, Zhang H. Schlemm's Canal and Trabecular Meshwork in Eyes with Primary Open Angle Glaucoma: A Comparative Study Using High-Frequency Ultrasound Biomicroscopy. PLoS One. 2016;11(1):e0145824-e
- ↑ Tandon A, Watson C, Ayyala R. Ultrasound biomicroscopy measurement of Schlemm's canal in pediatric patients with and without glaucoma. J aapos. 2017;21(3):234-7
- ↑ Fernández-Vigo JI, Kudsieh B, De-Pablo-Gómez-de-Liaño L, Almorín-Fernández-Vigo I, Fernández-Vigo C, García-Feijóo J, et al. Schlemm's canal measured by optical coherence tomography and correlation study in a healthy Caucasian child population. Acta Ophthalmol. 2019;97(4):e493-e8
- ↑ Chen Z, Sun J, Li M, Liu S, Chen L, Jing S, et al. Effect of age on the morphologies of the human Schlemm's canal and trabecular meshwork measured with swept‑source optical coherence tomography. Eye (Lond). 2018;32(10):1621-8
- ↑ Hong J, Xu J, Wei A, Wen W, Chen J, Yu X, et al. Spectral-domain optical coherence tomographic assessment of Schlemm's canal in Chinese subjects with primary open-angle glaucoma. Ophthalmology. 2013;120(4):709-15
- ↑ Chen J, Huang H, Zhang S, Chen X, Sun X. Expansion of Schlemm's canal by travoprost in healthy subjects determined by Fourier-domain optical coherence tomography. Invest Ophthalmol Vis Sci. 2013;54(2):1127-34
- ↑ Skaat A, Rosman MS, Chien JL, Mogil RS, Ren R, Liebmann JM, et al. Effect of Pilocarpine Hydrochloride on the Schlemm Canal in Healthy Eyes and Eyes With Open-Angle Glaucoma. JAMA Ophthalmol. 2016;134(9):976-81
- ↑ Park JH, Chung HW, Yoon EG, Ji MJ, Yoo C, Kim YY. Morphological changes in the trabecular meshwork and Schlemm's canal after treatment with topical intraocular pressure-lowering agents. Sci Rep. 2021;11(1):18169
- ↑ Rosman MS, Skaat A, Chien JL, Ghassibi MP, Sarimiye TF, Ritch R, et al. Effect of Cyclopentolate on In Vivo Schlemm Canal Microarchitecture in Healthy Subjects. J Glaucoma. 2017;26(2):133-7
- ↑ Skaat A, Rosman MS, Chien JL, Ghassibi MP, Liebmann JM, Ritch R, et al. Microarchitecture of Schlemm Canal Before and After Selective Laser Trabeculoplasty in Enhanced Depth Imaging Optical Coherence Tomography. J Glaucoma. 2017;26(4):361-6
- ↑ Varshney T, Azmira K, Gupta S, Mahalingam K, Singh A, Angmo D, et al. In Vivo Imaging of the Schlemm's Canal and the Response to Selective Laser Trabeculoplasty. Am J Ophthalmol. 2022;234:126-37
- ↑ Kuerten D, Plange N, Becker J, Walter P, Fuest M. Evaluation of Long-term Anatomic Changes Following Canaloplasty With Anterior Segment Spectral-domain Optical Coherence Tomography and Ultrasound Biomicroscopy. J Glaucoma. 2018;27(1):87-93
- ↑ Hong J, Yang Y, Wei A, Deng SX, Kong X, Chen J, et al. Schlemm's canal expands after trabeculectomy in patients with primary angle-closure glaucoma. Invest Ophthalmol Vis Sci. 2014;55(9):5637-42
- ↑ Elhusseiny AM, Jamerson EC, Menshawey R, Tam EK, El Sayed YM. Collector Channels: Role and Evaluation in Schlemm's Canal Surgery. Curr Eye Res. 2020;45(10):1181-1187.
- ↑ Li P, Butt A, Chien JL, Ghassibi MP, Furlanetto RL, Netto CF, et al. Characteristics and variations of in vivo Schlemm's canal and collector channel microstructures in enhanced-depth imaging optical coherence tomography. Br J Ophthalmol. 2017;101(6):808-13
- ↑ Meyer PA, Watson PG. Low dose fluorescein angiography of the conjunctiva and episclera. British Journal of Ophthalmology. 1987;71(1):2-10
- ↑ Akagi T, Uji A, Huang AS, Weinreb RN, Yamada T, Miyata M, et al. Conjunctival and Intrascleral Vasculatures Assessed Using Anterior Segment Optical Coherence Tomography Angiography in Normal Eyes. Am J Ophthalmol. 2018;196:1-9